Abstract
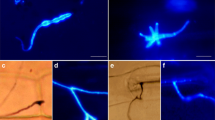
The response of parsley seedlings (Petroselinum crispum) inoculated with zoospores of the soybean-pathogenic fungus, Phytophthora megasperma f. sp. glycinea, ranged from “immunity” to “physiological susceptibility” depending on the post-inoculation environmental conditions. Typical nonhost resistance reactions, hypersensitive cell death and the formation of small local lesions, occurred under high relative humidity and 16 h illumination per day. Localized biochemical reactions were monitored using fluorescence microscopy combined with histochemical and immunohistochemical methods. The rapid accumulation of furanocoumarin phytoalexins, wall-bound phenolics and callose was detected around infection sites. Indirect antibody staining of frozen tissue sections demonstrated the local accumulation of phenylalanine ammonia-lyase, a key enzyme of general phenylpropanoid metabolism, and S-adenosyl-L-methionine: bergaptol O-methyltransferase, a specific enzyme of the furanocoumarin pathway. The results indicate that phenylpropanoid derivatives are synthesized de novo at infection sites.
Similar content being viewed by others
Abbreviations
- BMT:
-
S-adenosyl-L-methionine:bergaptol O-methyltransferase
- PAL:
-
phenylalanine ammonia-lyase
- PBS:
-
phosphate-buffered saline
References
Aist, J.R. (1976) Papillae and related wound plugs of plant cells. Annu. Rev. Phytopathol. 14, 145–163
Bailey, J.A. (1983) Biology of host-pathogen interactions. In: The dynamics of host defence. pp. 1–32, Bailey, J.A., Deverall, B.J., eds. Academic Press, Sydney New York London
Bell, A.B. (1981) Biochemical mechanisms of disease resistance. Annu. Rev. Plant Physiol. 32, 21–81
Burnette, N. (1981) “Western Blotting”: Electrophoretic transfer of proteins from sodium dodecylsulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Analyt. Biochem. 112, 195–203
Clark, G. (1981) Staining procedures, 4th edn. Williams and Williams, Baltimore and London
Eschrich, W., Currier, H.B. (1964) Identification of callose by its diachrome and fluorochrome reaction. Stain Technol. 39, 303–307
Feder, N., O'Brien, T. (1968) Plant microtechnique, some principles and new methods. Am. J. Bot. 55, 123–142
Fernandez, M.R., Heath, M.C. (1968) Cytological responses induced by five phytopathogenic fungi in a nonhost plant, Phaseolus vulgaris. Can. J. Bot. 54, 648–657
Giloh, H., Sedat, J.W. (1982) Fluorescence microscopy: reduced photobleaching of Rhodamine and Fluoresceine protein conjugates by n-propyl gallate. Science 217, 1252–1255
Hahlbrock, K., Cuypers, B., Douglas, C., Fritzemeier, K.H., Hoffmann, H., Rohwer, F., Scheel, D., Schulz, W. (1986) Biochemical interactions of plants with potentially pathogenic fungi. In: NATO ASI Series, vol. H4: Recognition in microbe-plant symbiotic and pathogenic interactions, pp. 311–323, Lugtenberg, B., ed. Springer, Berlin Heidelberg New York
Hahlbrock, K., Scheel, D. (1987) Biochemical responses of plants to pathogens. In: Innovative approaches to plant disease control, pp. 229–254, Chet, I., ed. John Wiley & Sons, New York
Hauffe, K.D., Hahlbrock, K., Scheel, D. (1986) Elicitor-stimulated biosynthesis in cultured parsley cells: S-adenosyl-L-methionine:bergaptol and S-adenosyl-L-methionine:xanthotoxol O-methyltransferases. Z. Naturforsch. 41c, 228–239
Heath, M.C. (1980) Reactions of nonsuscepts to fungal pathogens. Annu. Rev. Phytopathol. 18, 211–236
Jahnen, W. (1986) Untersuchungen zur Histochemie und Biochemie der Nichtwirt-Pathogenbeziehung von Petersilie (Petroselinum crispum, Hoffm.) und Phytophthora megasperma f. sp. glycinea. Dissertation, Universität zu Köln (F.R.G.)
Jensen, W.A. (1960) Botanical histochemistry, Freeman, San Franscisco
Knogge, W., Kombrink, E., Schmelzer, E., Hahlbrock, K. (1987) Phytoalexins and other putative defence-related substances in uninfected parsley plants. Planta 171, 279–287
Kombrink, E., Hahlbrock, K. (1986) Responses of cultured parsley cells to elicitors from phytopathogenic fungi. Plant Physiol. 81, 216–221
Kombrink, E., Bollmann, J., Hauffe, K.D., Knogge, W., Scheel, D., Schmelzer, E., Somssich I., Hahlbrock, K. (1986) Biochemical responses of non-host plant cells to fungi and fungal elicitors. In: Biology and molecular biology of plant-pathogen interactions, pp. 253–262, Bailey, J.A., ed. Plenum, New York London
Miller, T.J., Stone, H.O. (1978) The rapid isolation of ribonuclease-free immunoglobulin G by protein A-sepharose affinity chromatography. J. Immunol. Methods 24, 111–125
Moesta, P., Grisebach, H. (1983) Immunohistochemical detection of Phytophthora megasperma f. sp. glycinea in situ. Eur. J. Cell Biol. 31, 167–169
Ride, J.P. (1975) Lignification in wounded wheat leaves in response to fungi and its possible role in resistance. Physiol. Plant Pathol. 5, 125–134
Scheel, D., Hauffe, K.D., Jahnen, W., Hahlbrock, K. (1986) Stimulation of phytoalexin formation in fugnus-infected plants and elicitor-treated cell cultures of parsley. In: NATO ASI Series, Vol. H4: Recognition in microbe-plant symbiotic and pathogenic interactions, pp. 325–331, Lugtenberg, B., ed. Springer, Berlin Heidelberg New York
Schröder, J., Betz, B., Hahlbrock, K. (1976) Light-induced enzyme synthesis in cell suspension culture of Petroselinum hortense. Demonstration in a heterologous cell-free system of rapid changes in the rate of phenylalanine ammonia-lyase synthesis. Eur. J. Biochem. 67, 527–541
Sherwood, R.T., Vance, C.P. (1976) Histochemistry of papillae formed in reed canarygrass leaves in response to noninfecting pathogenic fungi. Phytopathology 66, 503–510
Sternberger, L. (1979) Immunocytochemistry, 2nd edn., John Wiley & Sons, New York Chichester Brisbane Toronto Singapore
Tietjen, K.G., Hunkler, D., Matern, U. (1983) Differntial response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. Eur. J. Biochem. 131, 401–407
Vance, C.P., Kirk, T.K., Sherwood, R.T. (1980) Lignification as a mechanism of disease resistance. Annu. Rev. Phytopathol. 18, 259–288
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jahnen, W., Hahlbrock, K. Cellular localization of nonhost resistance reactions of parsley (Petroselinum crispum) to fungal infection. Planta 173, 197–204 (1988). https://doi.org/10.1007/BF00403011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00403011