Summary
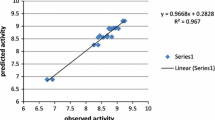
Quantitative structure-activity relationships (QSARs) for 40 HIV-1 inhibitors, 1-[(2-hydroxyethoxy)-methyl]-6-(phenylthio)thymine and its derivatives, were studied. Fully optimized geometries, based on the semiempirical AM1 method, were used to calculate electronic and molecular properties of all compounds. In order to examine the relation between biological activities and structural properties, multiple linear regression models were employed. A suitable QSAR model was obtained, showing not only statistical significance, but also predictive ability. The significant molecular descriptors used were atomic charges of two substituted carbon atoms in the thymine ring, hydration energies and molar refractivities of the molecules. These descriptors allowed a physical explanation of electronic and molecular properties contributing to HIV-1 inhibitory potency.
Similar content being viewed by others
References
Broder, S. (Ed.) AIDS, Modern Concepts and Therapeutic Challenges, Marcel Dekker, New York, NY, 1987.
Roey, P.V., Salerno, J.M., Duax, W.L., Chu, C.K., Ahn, M.K. and Schinazi, R.F., J. Am. Chem. Soc., 110 (1988) 2277.
Greengrass, C.W., Hoople, D.W.T., Street, S.D.A., Hamilton, F., Marriott, M.S., Bordner, J., Dalgleish, A.G., Mitsuya, H. and Broder, S., J. Med. Chem., 32 (1989) 618.
Tucker, T.J., LummaJr., W.C., Payne, L.S., Wai, J.M., de Solms, S.J., Giuliani, E.A., Darke, P.L., Hembach, J.C., Zugay, J.A., Schleif, W.A., Quintero, J.C., Emini, E.A., Huff, J.R. and Anderson, P.S., J. Med. Chem., 35 (1992) 2525.
Getman, D.P., DeCrescenzo, G.A., Heintz, R.M., Reed, K.L., Talley, J.J., Brayant, M.L., Celare, M., Houseman, K.A., Marr, J.J., Mueller, R.A., Vazquez, M.L., Shieh, H.-S., Stallings, W.C. and Stegeman, R.A., J. Med. Chem., 36 (1993) 288.
Clercq, E.D., J. Med. Chem., 38 (1995) 2491.
Tanaka, H., Takashima, H., Ubasawa, M., Sekiya, K., Nitta, I., Baba, M., Shigeta, S., Walker, R.T., Clercq, E. and Miyasaka, T., J. Med. Chem., 35 (1992) 337.
Tanaka, H., Takashima, H., Ubasawa, M., Sekiya, K., Nitta, I., Baba, M., Shigeta, S., Walker, R.T., Clercq, E. and Miyasaka, T., J. Med. Chem., 35 (1992) 4713.
Nicklaus, M.C., Milne, G.W.A. and BurkeJr., T.R., J. Comput.-Aided Mol. Design, 6 (1992) 487.
Klebe, G. and Abraham, U., J. Med. Chem., 36 (1993) 70.
Waller, C.L., Oprea, T.I., Giolitti, A. and Marshall, G.R., J. Med. Chem., 36 (1993) 4152.
Van de Waterbeemd, H. (Ed.) Advanced Computer-Assisted Techniques in Drug Discovery, VCH, Weinheim, 1994.
Kokpol, S.U., Hannongbua, S.V., Thongrit, N., Polman, S., Rode, B.M. and Swendinger, M.G., Anal. Sci., 4 (1988) 565.
Rode, B.M., Swendinger, M.G., Kokpol, S.U., Hannongbua, S.V. and Polman, S., Monatsschr. Chem., 120 (1989) 913.
Polman, S., Kokpol, S.U., Hannongbua, S.V. and Rode, B.M., Anal. Sci., 5 (1989) 641.
Hannongbua, S., In Proceedings of the 33rd Annual Conference of the Kasetsart University, Bangkok, January 30–February 1, 1995, pp. 273–279.
Pauling, L. and Pressman, D., J. Am. Chem. Soc., 67 (1945) 1003.
Agin, D., Hersh, L. and Holtzman, D., Proc. Natl. Acad. Sci. USA, 53 (1965) 952.
Silipo, C. and Hansch, C., J. Am. Chem. Soc., 97 (1975) 6849.
Selassie, C.D., Fang, S.-X., Li, R., Hansch, C., Debnath, G., Klien, T.E., Langridge, R. and Kaufman, B.T., J. Med. Chem., 32 (1989) 1895.
Andrea, T.A. and Kalayeh, H., J. Med. Chem., 34 (1991) 2824.
Ferguson, D.M., Radmer, R.J. and Kollman, P.A., J. Med. Chem., 34 (1991) 2654.
Reddy, M.R., Vishwanadhan, V.N. and Weinstein, J.N., Proc. Natl. Acad. Sci. USA, 88 (1991) 10287.
Lybrand, T.P. and McCammon, J.A., J. Comput.-Aided Mol. Design, 2 (1988) 259.
Hansch, C. and Fujita, T., J. Am. Chem. Soc., 86 (1964) 1616.
Hansch, C. and Leo, A., Substituent Constants for Correlation Analysis in Chemistry and Biology, Wiley, New York, NY, 1979.
Verloop, A., In Ariëns, E.J. (Ed.) Drug Design, Academic Press, New York, NY, 1972.
ALCHEMY III, Tripos Associates Inc., St. Louis, MO, 1992.
GAUSSIAN 92, Gaussian Inc., Pittsburgh, PA, 1992.
Dewar, M.J., Zoebisch, E.G., Healy, E.F. and Stewart, J.P., J. Am. Chem. Soc., 107 (1985) 3902.
ChemPlus, Hypercube Inc., Waterloo, ON, 1993.
Ghose, A.K. and Crippen, G.M., J. Chem. Inf. Comput. Sci., 27 (1987) 21.
Viswanadhan, V.N., Ghose, A.K., Revankar, G.N. and Robins, R.K., J. Chem. Inf. Comput. Sci., 29 (1989) 163.
Bodor, N., Gabanyi, Z. and Wong, C., J. Am. Chem. Soc., 111 (1989) 3783.
Still, W.C., Tempczyk, A., Hawley, R.C. and Hendrickson, T., J. Am. Chem. Soc., 112 (1990) 6127.
Norusis, M.J., The SPSS Guide to Data Analysis for SPSS/PC+, 2nd ed., SPSS, Inc., Chicago, IL, 1991.
Cramer, R.D., Bunce, J.D. and Patterson, D.E., Quant. Struct.-Act. Relatsh., 7 (1988) 18.
Topliss, J.G. and Costello, R.J., J. Med. Chem., 15 (1973) 1066.
Wold, S., Quant. Struct.-Act. Relatsh., 10 (1991) 191.
Kubinyi, H., QSAR: Hansch Analysis and Related Approaches, VCH, Weinheim, 1993.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hannongbua, S., Lawtrakul, L. & Limtrakul, J. Structure-activity correlation study of HIV-1 inhibitors: Electronic and molecular parameters. J Computer-Aided Mol Des 10, 145–152 (1996). https://doi.org/10.1007/BF00402822
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00402822