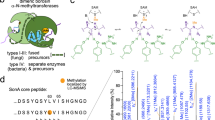
Abstract
Several newly reported post-translational modification reactions are involved in lantibiotic biosynthesis. A short overview of the present knowledge on the post-translational modifications and on the enzymes involved in lantibiotic biosynthesis is given. The oxidative decarboxylation of the epidermin precursor peptide EpiA is described in detail. The FMN-containing oxidoreductase EpiD is involved in the formation of the C-terminal S-[(Z)-2-aminovinyl]-D-cysteine residue of epidermin: under reducing conditions the side chain of the C-terminal cysteine residue of EpiA is converted to an enethiol. EpiD has no absolute substrate specificity and can be used for modification of peptides having the C-terminal consensus motif [V/I/L/(M)/F/Y/W]-[A/S/V/T/C/(I/L)]-C.
Similar content being viewed by others
Abbreviations
- Dha:
-
2,3-didehydroalanine
- Dhb:
-
(Z)-2,3-didehydrobutyrine
- ES-MS:
-
Electrospray Mass Spectrometry
- FAD:
-
Flavin Adenine Dinucleotide
- FMN:
-
Flavin Mononucleotide
- MBP:
-
Maltose-Binding Protein
- TFA:
-
TrifluoroAcetic Acid
- TLC:
-
Thin-Layer Chromatography
References
Allgaier H, Jung G, Werner RG, Schneider U & Zähner H (1985) Elucidation of the structure of epidermin, a ribosomally synthesized, tetracyclic heterodetic polypeptide antibiotic. Angew. Chem. Int. Ed. Engl. 24: 1051–1053
Allgaier H, Jung G, Werner RG, Schneider U & Zähner H (1986) Epidermin: sequencing of a heterodet tetracyclic 21-peptide amide antibiotic. Eur. J. Biochem. 160: 9–22
Augustin J, Rosenstein R, Kupke T, Schneider U, Schnell N, Engelke G, Entian K-D & Götz F (1991) Identification of epidermin biosynthetic genes by complementation studies and heterologous expression. In: Jung G & Sahl H-G (Ed) Nisin and novel lantibiotics (pp 277–286). Escom, Leiden
Augustin J, Rosenstein R, Wieland B, Schneider U, Schnell N, Engelke G, Entian K-D & Götz F (1992) Genetic analysis of epidermin biosynthetic genes and epidermin-negative mutants of Staphylococcus epidermidis. Eur. J. Biochem. 204: 1149–1154
Banerjee S & Hansen JN (1988) Structure and expression of a gene encoding the precursor of subtilin, a small protein antibiotic. J. Biol. Chem. 263: 9508–9514
Bayer A, Freund S, Nicholson G & Jung G (1993) Posttranslational backbone modifications in the ribosomal biosynthesis of the glycine-rich antibiotic microcin B17. Angew. Chem. Int. Ed. Engl. 32: 1336–1339
Beck-Sickinger AG & Jung G (1991) Synthesis and conformational analysis of lantibiotic leader-, pro- and pre-peptides. In: Jung G & Sahl H-G (Eds) Nisin and novel lantibiotics (pp 218–230). Escom, Leiden
Beck-Sickinger AG & Jung G (1993) Synthesis and conformational-analysis by CD spectroscopy of lantibiotic leader, pro-peptides and pre-peptides. Liebigs Annalen der Chemie 1125–1131
Biemann K (1990) Sequencing of peptides by tandem mass spectrometry and high-energy collision-induced dissociation. Methods. Enzymol. 193: 455–479
Bierbaum G, Reis M, Szekat C & Sahl H-G (1994) Construction of an expression system for engineering of the lantibiotic Pep5. Appl. Environ. Microbiol. 60: 4332–4338
Breil BT, Ludden PW & Triplett EW (1993) DNA Sequence and mutational analysis of genes involved in the production and resistance of the antibiotic peptide trifolitoxin. J. Bacteriol. 175: 3693–3702
Buchman GW, Banerjee S & Hansen JN (1988) Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J. Biol. Chem. 263: 16260–16266
Bycroft BW (1969) Structural relationships in microbial peptides. Nature 224: 595–597
Bycroft BW, Chan WC & Roberts GCK (1991) Synthesis and characterization of pro- and prepeptides related to nisin and subtilin. In: Jung G & Sahl H-G (Eds) Nisin and novel lantibiotics (pp 204–217). Escom, Leiden
Chan WC, Bycroft BW, Lian L-X & Roberts GCK (1989) Isolation and characterization of two degradation products derived from the peptide antibiotic nisin. FEBS Letters 252: 29–36
Chan WC, Bycroft BW, Leyland ML, Lian L-Y & Roberts GCK (1993) A novel post-translational modification of the peptide antibiotic subtilin: isolation and characterization of a natural variant from Bacillus subtilis ATCC 6633. Biochem. J. 291: 23–27
Davagnino J, Herrero M, Furlong D, Moreno F & Kolter R (1986) The DNA replication inhibitor microcin B17 is a forty-three amino acid protein containing sixty percent glycine. Proteins 1: 230–238
Engelke G, Gutowski-Eckel Z, Hammelmann M & Entian KD (1992) Biosynthesis of the lantibiotic nisin: genomic organization and membrane localization of the NisB protein. Appl. Environ. Microbiol. 58: 3730–3743
Fredenhagen A, Märki F, Fendrich G, Märki W, Gruner J, Oostrum Jv, Raschdorf F & Peter HH (1991) Duramycin B and C, two new lanthionine-containing antibiotics as inhibitors of phospholipase A2, and structural revision of duramycin and cinnamycin. In: Jung G & Sahl H-G (Eds) Nisin and novel lantibiotics (pp 131–140). Escom, Leiden
Genet R, Denoyelle C & Ménez A (1994) Purification and partial characterization of an amino acid α,β-dehydrogenase. L-tryptophan 2′,3′-oxidase from Chromobacterium violaceum. J. Biol. Chem. 269: 18177–18184
Genilloud O, Moreno F & Kolter R (1989) DNA sequence, products, and transcriptional pattern of the genes involved in production of the DNA replication inhibitor microcin B17. J. Bacteriol. 171: 1126–1135
Ghisla S & Massey V (1989) Mechanisms of flavoprotein-catalyzed reactions. Eur. J. Biochem. 181: 1–17
Gilmore MS, Segarra RA, Booth MC, Bogie CP, Hall LR & Clewell DB (1994) Genetic structure of the Enterococcus faecalis plasmid pAD1-encoded cytolytic toxin system and its relationship to lantibiotic determinants. J. Bacteriol. 176: 7335–7344
Grabowski R & Buckel W (1991) Purification and properties of an iron-sulfur-containing and pyridoxal-phosphate-independent L-serine dehydratase from Peptostreptococcus asaccharolyticus. Eur. J. Biochem. 199: 89–94
Grabowski R, Hofmeister AEM & Buckel W (1993) Bacterial l-serine dehydratases: a new family of enzymes containing iron-sulfur clusters. Trends Biochem. Sci. 18: 297–300
Gross E & Morell JL (1967) The presence of dehydroalanine in the antibiotic nisin and its relationship to activity. J. Am. Chem. Soc. 89: 2791–2792
Gross E & Morell JL (1969) Dehydroalanyl-lysine: identical COOH-terminal structures in peptide antibiotics nisin and subtilin. Proc. Natl. Acad. Sci. USA 62: 952–956
Gross E & Morell JL (1971) The structure of nisin. J. Am. Chem. Soc. 93: 4634–4635
Gross E & Kiltz HH (1973) The number and nature of α,β-unsaturated amino acids in subtilin. Biochem. Biophys. Res. Commun. 50: 559–565
Heck SD, Siok CJ, Krapcho KJ, Kelbaugh PR, Thadeio PF, Welch MJ, Williams RD, Ganong AH, Kelly ME, Lanzetti AJ, Gray WR, Phillips D, Parks TN, Jackson H, Ahlijanian MK, Saccomano NA & Volkmann RA (1994) Functional consequences of posttranslational isomerization of Ser46 in a calcium channel toxin. Science 266: 1065–1068
Ingram LC (1969) Synthesis of the antibiotic nisin: Formation of lanthionine and β-methyllanthionine. Biochim. Biophys. Acta 184: 216–219
Ingram L (1970) A ribosomal mechanism for synthesis of peptides related to nisin. Biochim. Biophys. Acta 224: 263–265
Jack RW, Carne A, Metzger J, Stefanovic S, Sahl H-G, Jung G & Tagg J (1994) Elucidation of the structure of SA-FF22, a lanthionine-containing antibacterial peptide produced by Streptococcus pyogenes strain FF22. Eur. J. Biochem. 220: 455–462
Kellner R, Jung G, Hörner T, Zähner H, Schnell N, Entian K-D & Götz F (1988) Gallidermin: a new lanthionine-containing polypeptide antibiotic. Eur. J. Biochem. 177: 53–59
Kellner R, Jung G, Josten M, Kaletta C, Entian K-D & Sahl H-G (1989) Pep5, a new lantibiotic: structure elucidation and amino acid sequence of the propeptide. Angew. Chem. Int. Ed. Engl. 28: 616–619
Kessler H, Steuernagel S, Will M, Jung G, Kellner R, Gillessen D & Kamiyama T (1988) The structure of the polycyclic nonadecapeptide Ro09-0198. Helv. Chim. Acta 71: 1924–1929
Klein C, Kaletta C, Schnell N & Entian K-D (1992) Analysis of genes involved in biosynthesis of the lantibiotic subtilin. Appl. Environ. Microbiol. 58: 132–142
Kleinkauf H & vonDöhren H (1987) Biosynthesis of peptide antibiotics. Annu. Rev. Microbiol. 41: 259–289
Kogler H, Bauch M, Fehlhaber H-W, Griesinger C, Schubert W & Teetz V (1991) NMR-spectroscopic investigation on mersacidin. In: Jung G & Sahl H-G (Eds) Nisin and novel lantibiotics (pp 159–170). Escom, Leiden
Kuipers OP, Rollema HS, Yap WMGJ, Boot HJ, Siezen RJ & deVos WM (1992) Engineering dehydrated amino acid residues in the antimicrobial peptide nisin. J. Biol. Chem. 267: 24340–24346
Kuipers OP, Beerthuyzen MM, Siezen RJ & deVos WM (1993) Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis: requirement of expression of the nisA and nisI genes for development of immunity. Eur. J. Biochem. 216: 281–291
Kupke T, Stevanovic S, Sahl H-G & Götz F (1992) Purification and characterization of EpiD, a flavoprotein involved in the biosynthesis of the lantibiotic epidermin. J. Bacteriol. 174: 5354–5361
Kupke T, Stevanovic S, Ottenwälder B, Metzger JW, Jung G & Götz F (1993) Purification and characterization of EpiA, the peptide substrate for posttranslational modifications involved in epidermin biosynthesis. FEMS Lett. 112: 43–48
Kupke T, Kempter C, Gnau V, Jung G & Götz F (1994) Mass spectroscopic analysis of a novel enzymatic reaction: oxidative decarboxylation of the lantibiotic precursor peptide EpiA catalyzed by the flavoprotein EpiD. J. Biol. Chem. 269: 5653–5659
Kupke T, Kempter C, Jung G & Götz F (1995) Oxidative decarboxylation of peptides catalyzed by flavoprotein EpiD: determination of substrate specificity using peptide libraries and neutral loss mass spectrometry. J. Biol. Chem. 270: 11282–11289
Langer M, Reck G, Reed J & Retey J (1994) Identification of serine-143 as the most likely precursor of dehydroalanine in the activesite of histidine ammonia-lyase — a study of the overexpressed enzyme by site-directed mutagenesis. Biochemistry 33: 6462–6467
Liu W & Hansen JN (1993) The antimicrobial effect of a structural variant of subtilin against outgrowing Bacillus cereus T spores and vegetative cells occurs by different mechanisms. Appl. Environ. Microbiol. 59: 648–651
Metzger JW, Kempter C, Wiesmüller K-H & Jung G (1994) Electrospray mass spectrometry and tandem mass spectrometry of synthetic multicomponent peptide mixtures: determination of composition and purity. Anal. Biochem. 219: 261–277
Meyer C, Bierbaum G, Heidrich C, Reis M, Süling J, Iglesias-Wind MI, Kempter C, Molitor E & Sahl H-G (1995) Nucleotide sequence of the lantibiotic Pep5 biosynthetic gene cluster and functional analysis of PepP and PepC: evidence for a role of PepC in thioethher formation. Eur. J. Biochem. 232: 478–489
Meyer HE, Heber M, Eisermann B, Korte H, Metzger JW & Jung G (1994) Sequence analysis of lantibiotics: chemical derivatization procedures allow a fast access to complete Edman degradation. Anal. Biochem. 223: 185–190
Mor A, Amiche M & Nicolas P (1992) Enter a new post-translational modification: d-amino acids in gene encoded peptides. Trends Biochem. Sci. 17: 481–485
Ottenwälder B, Kupke T, Brecht S, Gnau V, Metzger J, Jung G & Götz F (1995) Isolation and characterization of geretically engineered gallidermin and epidermin analogs. Appl. Environ. Microbiol. 61: 3894–3903
Peschel A, Augustin J, Kupke T, Stevanovic S & Götz F (1993) Regulation of epidermin biosynthetic genes by EpiQ. Mol. Microbiol. 9: 31–39
Piard J-C, Kuipers OP, Rollema HS, Desmazeaud MJ & deVos W (1993) Structure, organization, and expression of the lct gene for lacticin 481, a novel lantibiotic produced by Lactococcus lactis. J. Biol. Chem. 268: 16361–16368
Richter K, Egger R & Kreil G (1987) D-Alanine in the frog skin peptide demorphin is derived from L-alanine in the precursor. Science 238: 200–202
Riggs PD (1990) Expression and purification of maltose-binding protein fusions. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA & Struhl K (Eds) Current protocols in molecular biology (pp 16.6.1–16.6.12). John Wiley and Sons, Inc., New York
Sahl H-G, Reis M, Eschbach M, Szekat C, Beck-Sickinger AG, Metzger J, Stevanovic S & Jung G (1991) Isolation of Pep5 prepeptides in different stages of modification. In: Jung G & Sahl H-G (Ed) Nisin and novel lantibiotics (pp 332–346). Escom, Leiden
Scaloni A, Barra D & Bossa F (1994) Sequence analysis of dehydroamino acid-containing peptides. Anal. Biochem. 218: 226–228
Schnell N, Entian K-D, Schneider U, Götz F, Zähner H, Kellner R & Jung G (1988) Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature 333: 276–278
Schnell N, Engelke G, Augustin J, Rosenstein R, Ungermann V, Götz F & Entian K-D (1992) Analysis of genes involved in the biosynthesis of lantibiotic epidermin. Eur. J. Biochem. 204: 57–68
Skaugen M, Nissen-Meyer J, Jung G, Stevanovic S, Sletten K, Mortvedt Abildgaard CI & Nes IF (1994) In vivo conversion of L-serine to D-alanine in a ribosomally synthesized polypeptide. J. Biol. Chem. 269: 27183–27185
Stachelhaus T & Marahiel MA (1995) Modular structure of genes encoding multifunctional peptide synthetases required for non-ribosomal peptide synthesis. FEMS Microbiol. Lett. 125: 3–14
Tabor S (1990) Expression using the T7 RNA polymerase/promoter system. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA & Struhl K (Eds) Current protocols in molecular biology (pp 16.2.1–16.2.11). John Wiley and Sons, Inc., New York
Takai K & Hayaishi O (1987) Purification and properties of tryptophan side chain oxidase types I and II from Pseudomonas. Methods. Enzymol. 142: 195–217
Toogood PL (1993) Model studies of lantibiotic biogenesis. Tetrahedron Letters 34: 7833–7836
Van deKamp M, Horstink LM, van denHooven HW, Konings RNH, Hilbers CW, Frey A, Sahl H-G, Metzger JW & van deVen FJM (1995) Sequence analysis by NMR spectroscopy of the peptide lantibiotic epilancin K7 from Staphylococcus epidermidis K7. Eur. J. Biochem. 227: 757–771
Van derMeer JR, Polman J, Beerthuyzen MM, Siezen RJ, Kuipers OP & deVos WM (1993) Characterization of the Lactococcus lactis nisin A operon genes nisP, encoding a subtilisin-like serine protease involved in precursor processing, and nisR, encoding a regulatory protein involved in nisin biosynthesis. J. Bacteriol 175: 2578–2588
Van derMeer JR, Rollema HS, Siezen RJ, Beerthuyzen MM, Kuipers OP & deVos WM (1994) Influence of amino acid substitutions in the nisin leader peptide on biosynthesis and secretion of nisin by Lactococcus lactis. J. Biol. Chem. 269: 3555–3562
Wakamiya T, Ueki Y, Shiba T, Kido Y & Motoki Y (1985) The structure of ancovenin, a new peptide inhibitor of angiotensin I converting enzyme. Tetrahedron Lett. 26: 665–668
Wakamiya T, Fukase K, Naruse N, Konishi M & Shiba T (1988) Lanthiopeptin, a new peptide effective against herpes simplex virus: structural determination and comparison with Ro 09-0198, an immunopotentiating peptide. Tetrahedron Lett. 29: 4771–4772
Weil HP, Beck-Sickinger AG, Metzger J, Stevanovic S, Jung G, Josten M & Sahl H-G (1990) Biosynthesis of the lantibiotic Pep 5: Isolation and characterization of a prepeptide containing dehydroamino acids. Eur. J. Biochem. 194: 217–223
Yan SCB, Grinnell BW & Wold F (1989) Post-translational modifications of proteins: some problems left to solve. Trends. Biochem. Sci. 14: 264–268
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kupke, T., Götz, F. Post-translational modifications of lantibiotics. Antonie van Leeuwenhoek 69, 139–150 (1996). https://doi.org/10.1007/BF00399419
Issue Date:
DOI: https://doi.org/10.1007/BF00399419