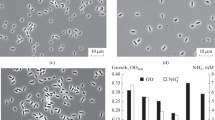
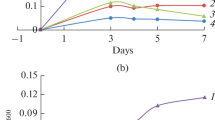
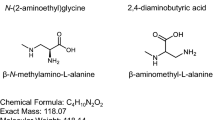
Abstract
Glycine betaine is accumulated as a compatible solute in many photosynthetic and non-photosynthetic bacteria — the last being unable to synthesize the compound - and thus large pools of betaine can be expected to be present in hypersaline environments. A variety of aerobic and anaerobic microorganisms degrade betaine to among other products trimethylamine and methylamine, in a number of different pathways. Curiously, very few of these betaine breakdown processes have yet been identified in hypersaline environments. Trimethylamine can also be formed by bacterial reduction of trimethylamine N-oxide (also by extremely halophilic archaeobacteria). Degradation of trimethylamine in hypersaline environments by halophilic methanogenic bacteria is relatively well documented, and leads to the formation of methane, carbon dioxide and ammonia.
Similar content being viewed by others
References
Barret EL & Kwan HS (1985) Bacterial reduction of trimethylamine oxide. Ann. Rev. Microbiol. 39: 131–149
Bernard T, Pocard JA, Perroud B & Le Rudulier D (1986) Variations in the response of salt-stressed Rhizobium strains to betaines. Arch. Microbiol. 143: 359–364
Delwiche CC & Bregoff HM (1958) Pathway of betaine and choline synthesis in Beta vulgaris. J. Biol. Chem. 223: 430–433
Gabbay-Azaria R, Tel-Or E & Schonfeld M (1988) Glycinebetaine as an osmoregulant and compatible solute in the marine cyanobacterium Spirulina subsalsa. Arch. Biochem. Biophys. 264: 333–339
Galinski EA & Trüper HG (1982) Betaine, a compatible solute in the extremely halophilic phototrophic bacterium Ectothiorhodospira halochloris. FEMS Microbiol. Letters 13: 357–360
Giani D, Giani L, Cohen Y & Krumbein WE (1985) Methanogenesis in the hypersaline Solar Lake (Sinai). FEMS Microbiol. Letters. 25: 219–224
Heijthuijsen JHFG & Hansen TA (1989a) Betaine fermentation and oxidation by marine Desulfuromonas strains. Appl. Environ. Microbiol. 154: 965–969
Heijthuijsen JHFG & Hansen TA (1989b) Anaerobic degradation of betaine by marine Desulfobacterium strains. Arch. Microbiol. 152: 393–396
Hippe H, Caspari D, Fiebig K & Gottschalk G (1979) Utilization of trimethylamine and other N-methyl compounds for growth and methane formation by Methanosarcina barkeri. Proc. Natl. Acad. Sci. USA 76: 494–498
Hormann K & Andreesen JR (1989) Reductive cleavage of sarcosine and betaine in Eubacterium acidaminophilum via enzyme systems different from glycine reductase. Arch. Microbiol. 153: 50–59
Ikuta S, Matuura K, Imamura S, Misaki H & Horiuti Y (1977) Oxidative pathway of choline to betaine in the soluble fraction prepared from Arthrobacter globiformis. J. Biochem. 82: 157–163
Imhoff JF & Rodriguez-Valera F (1984) Betaine is the main compatible solute of halophilic eubacteria. J. Bacteriol. 160: 478–479
King GM (1984) Metabolism of trimethylamine, choline, and glycine betaine by sulfate-reducing and methanogenic bacteria in marine sediments. Appl. Environ Microbiol. 48: 719–725
King GM (1988) Methanogenesis from methylated amines in a hypersaline algal mat. Appl. Environ. Microbiol. 54: 130–136
Large PJ & Green J (1984) Oxidation of mono- di-, and trimethylamine by methazotrophic yeasts: properties of the microsomal and peroxisomal enzymes involved and comparison with bacterial enzyme systems. In: Crawford RL & Hanson RS (Eds) Microbial Growth on C1 Compounds (pp 155–164). American Society for Microbiology, Washington, D.C.
Le Rudulier D & Bernard T (1986) Salt tolerance in Rhizobium: a possible role for betaines. FEMS Microbiol. Rev. 39: 67–72
Le Rudulier D & Bouillard L (1983) Glycine betaine, an osmotic effector in Klebsiella pneumoniae and other members of the Enterobacteriaceae. Appl. Environ. Microbiol. 46: 152–159
Mackay MA, Norton RS & Borowitzka LJ (1984) Organic osmoregulatory solutes in cyanobacteria. J. Gen. Microbiol. 130: 2177–2191
Madigan MT, Cox JC & Gest H (1980) Physiology of dark fermentative growth of Rhodopseudomonas capsulata. J. Bacteriol. 142: 908–915
Mathrani IM & Boone DR (1985) Isolation and characterization of a moderately halophilic methanogen from a solar saltern. Appl. Environ. Microbiol. 50: 140–143
Mohammad FAA, Reed RH & Stewart WDP (1983) The halophilic cyanobacterium Synechocystis DUN52 and its osmotic responses. FEMS Microbiol. Letters 16: 287–290
Möller B, Oßmer R, Howard BH, Gottschalk G & Hippe H (1984) Sporomusa, a new genus of gram-negative anaerobic bacteria including Sporomusa sphaeroides spec. nov. and Sporomusa ovata spec. nov. Arch. Microbiol. 139: 388–396
Moore DJ, Reed RH & Stewart WDP (1987) A glycine betaine transport system in Aphanothece halophytica and other glycine betaine synthesising cyanobacteria. Arch. Microbiol. 147: 399–405
Müller E, Fahlbusch K, Walther R & Gottschalk G (1981) Formation of N,N-dimethylglycine, acetic acid and butyric acid from betaine by Eubacterium limosum. Appl. Environ. Microbiol. 42: 439–445
Naumann E, Hippe H & Gottschalk G (1983) Betaine: new oxidant in the Stickland reaction and methanogenesis from betaine and L-alanine by a Clostridium sporogenes-Methanosarcina barkeri coculture. Appl. Environ. Microbiol. 45: 474–483
Nicolaus B, Lanzotti V, Trincone A, De Rosa M, Grant WD & Gambacorta A (1989) Glycine betaine and polar lipid composition in halophilic archaebacteria in response to growth in different salt concentrations. FEMS Microbiol. Letters 59: 157–160
Oremland RS (1988) Biogeochemistry of methanogenic bacteria. In: Zehnder AJB (Ed) Biology of Anaerobic Microorganisms (pp 641–706). John Wiley & Sons, New York
Oremland RS & King GM (1989) Methanogenesis in hypersaline environments. In: Cohen Y & Rosenberg E (Eds) Microbial Mats. Physiological Ecology of Benthic Microbial Communities (pp 180–190). American Society for Microbiology, Washington, D.C.
Oren A (1988) Anaerobic degradation of organic compounds at high salt concentrations. Antonie v. Leeuwenhoek 54: 267–277
Oren A (1989) Photosynthetic and heterotrophic benthic bacterial communities of a hypersaline sulfur spring on the shore of the Dead Sea (Hamei Mazor). In: Cohen Y & Rosenberg E (Eds) Microbial Mats. Physiological Ecology of Benthic Microbial Communities (pp 64–76). American Society for Microbiology, Washington, D.C
Oren A, Kessel M & Stackebrandt E (1989) Ectothiorhodospira marismortui sp. nov., an obligately anaerobic, moderately halophilic purple sulfur bacterium from a hypersaline sulfur spring on the shore of the Dead Sea. Arch. Microbiol. 151: 524–529
Oren A, Pohla H & Stackebrandt E (1987) Transfer of Clostridium lortetii to a new genus Sporohalobacter gen. nov. as Sporohalobacter lortetii comb. nov., and description of Sporohalobacter marismortui. System. Appl. Microbiol. 9: 239–246
Oren A & Trüper HG (1990) Anaerobic growth of halophilic archaeobacteria by reduction of dimethylsulfoxide and trimethylamine N-oxide. FEMS Microbiol. Letters 70: 33–36
Paterek JR & Smith PH (1985) Isolation and characterization of a halophilic methanogen from Great Salt Lake. Appl. Environ. Microbiol. 52: 877–881
Rafaeli-Eshkol D & Avi-Dor Y (1968) Studies in halotolerance in a moderately halophilic bacterium. Effect of betaine on salt resistance of the respiratory system. Biochem. J. 109: 687–691
Reed RH, Borowitzka LJ, Mackay MA, Chudek JA, Foster R, Warr SRC, Moore DJ & Stewart WDP (1986) Organic solute accumulation in osmotically stressed cyanobacteria. FEMS Microbiol. Rev. 39: 51–56
Robertson DE, Noll D, Roberts MF, Menaia JAGF & Boone DR (1990) Detection of the osmoregulator betaine in methanogens. Appl. Environ. Microbiol. 56: 563–565
Roth WG, Leckie MP & Dietzler DN (1988) Restoration of colony-forming activity in osmotically stressed Escherichia coli by betaine. Appl. Environ. Microbiol. 54: 3142–3146
Schweinfurth G & Lewin L (1898) Beiträge zur Topographie und Geochemie des ägyptischen Natron-Thals. Zeitschr. d. Ges. f. Erdk. 33: 1–25
Strøm AR, Olafsen JA & Larsen H (1979) Trimethylamine oxide: a terminal electron acceptor in anaerobic respiration of bacteria. J. Gen. Microbiol. 112: 315–320
Trüper HG & Galinski EA (1989) Compatible solutes in halophilic phototrophic procaryotes. In: Cohen Y & Rosenberg E (Eds) Microbial Mats. Physiological Ecology of Benthic Microbial Communities (pp 342–348). American Society for Microbiology, Washington, D.C.
Tschichholz I & Trüper HG (1990) Fate of compatible solutes during dilution stress in Ectothiorhodospira halochloris. FEMS Microb. Ecol. 73: 181–186
Wohlfarth A, Severin J & Galinski EA (1990) The spectrum of compatible solutes in heterotrophic halophilic eubacteria of the family Halomonadaceae. J. Gen. Microbiol. 136: 705–712
Yancey PH, Clark ME, Hand SC, Bowlus RD & Somero GN (1982) Living with water stress: Evolution of osmolyte systems. Science 217: 1214–1222
Zhilina TN (1986) Methanogenic bacteria from hypersaline environments. System. Appl. Microbiol. 7: 216–222
Zhilina TN & Zavarzin GA (1990a) A new extremely halophilic homoacetogen bacteria Acetohalobium arabaticum, gen. nov., sp. nov. Dokl. Akad. Nauk. SSSR 311: 745–747 (in Russian)
Zhilina TN & Zavarzin GA (1990b) Extremely halophilic, methylotrophic, anaerobic bacteria. FEMS Microbiol. Rev. (in press)
Zindel U, Freudenberg W, Rieth M, Andreesen JR, Schnell J & Widdel F (1988) Eubacterium acidaminophilum sp. nov., a versatile amino acid-degrading anaerobe producing or utilizing H2 or formate. Description and enzymatic studies. Arch. Microbiol. 150: 254–266
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oren, A. Formation and breakdown of glycine betaine and trimethylamine in hypersaline environments. Antonie van Leeuwenhoek 58, 291–298 (1990). https://doi.org/10.1007/BF00399342
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00399342