Summary
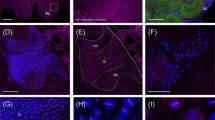
Honeybee embryos were stained with a monoclonal antibody raised against the Drosophila engrailed protein. The antibody was found to label rows of nuclei in the transverse grooves that form the earliest external sign of metameric germ band organization. These grooves demarcate metameric units about seven cell rows wide, of which about three rows with reduced apical cell surfaces account for the grooves. The en stripes appear in the grooves as soon as these form and grow from one to about four cells in width and thus completely overlap the groove. During the rudimentary germ band retraction, the grooves shift slightly backwards relative to both the en stripes and the trachdeal pits. The spatio-temporal pattern by which the series of grooves and stripes arises is quite striking. Both become visible first in the gnathal and thoracic regions, then in the pregnathal parts of the head and in the abdomen. The stripes arise essentially in an antero-posterior sequence. In addition, the earliest stripes to form display a pattern of alternating intensities whereas the later stripes, those in the abdomen, arise with approximately equal strength. The latter trait was earlier observed in the grasshopper, while the former is known from Drosophila where, however, the strong stripes correspond to the weak stripes in the honeybee.
Similar content being viewed by others
References
Akam M (1987) The molecular basis for metameric pattern in the Drosophila embryo. Development 101:1–22
Beeman RW, Stuart JJ, Haas MS, Denell RE (1989) Genetic analysis of the homeotic gene complex (HOM-C) in the beetle Tribolium castaneum. Dev Biol 133:196–209
Carroll SB, DiNardo S, O'Farrell PH, White RAH, Scott MP (1988) Temporal and spatial relationships between segmentation and homeotic gene expression in Drosophila embryos: distributions of the fushi tarazu, engrailed, Sex combs reduced, Antennapedia, and Ultrabithorax proteins. Genes Dev 2:350–360
DiNardo S, O'Farrell P (1989) Establishment and refinement of segmental pattern in the Drosophila embryo: spatial control of engrailed expression by pair-rule genes. Genes Dev 1:1212–1225
DiNardo S, Kuner JM, Theis J, O'Farrell PH (1985) Development of embryonic pattern in Drosophila melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell 43:59–69
Fleig R, Sander K (1986) The embryogenesis of the honeybee Apis mellifera L. (Hymenoptera): a SEM study. Int J Insect Morphol Embryol 15:449–462
Fleig R, Walldorf U, Gehring WJ, Sander K (1988) In situ localization of the transcripts of a homeobox gene in the honeybee Apis mellifera L. (Hymenoptera). Roux's Arch Dev Biol 197:269–274
Foe VE (1989) Mitotic domains reveal early commitment of cells in Drosophila embryos. Development 107:1–22
Ingham PW (1988) The molecular genetics of embryonic pattern formation in Drosophila. Nature 335:25–34
Karr TL, Weir MP, Ali Z, Kornberg T (1989) Patterns of engrailed expression in early Drosophila embryos. Development 105:605–612
Kornberg T, Sidén I, O'Farrell PH, Simon M (1985) The engrailed locus of Drosophila: in situ localization of transcripts reveals compartment-specific expression. Cell 40:45–53
Krause G (1939) Die Eitypen der Insekten. Biol Zentralbl 59:495–536
Lawrence PA (1981) The cellular basis of segmentation in insects. Cell 26:3–10
Lawrence PA (1988) The present status of the parasegment. Development 104 [Suppl]:61–65
Lawrence PA, Johnston P (1989) Pattern formation in the Drosophila embryo: allocation of cells to parasegments by even-skipped and fushi tarazu. Development 105:761–767
Lawrence PA, Johnston P, Macdonald P, Struhl G (1987) Borders of parasegments in Drosophila embryo are delimited by the fushi tarazu and even-skipped genes. Nature 328:440–442
Martinez-Arias A, Lawrence PA (1985) Parasegments and compartments in the Drosophila embryo. Nature 313:639–642
Nüsslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287:795–801
Nüsslein-Volhard C, Wieschaus E, Jürgens G (1982) Segmentierung bei Drosophila —eine genetische Analyse. Verh Dtsch Zool Ges 1982:91–104
Patel NH, Kornberg TB, Goodman CS (1989) Expression of engrailed during segmentation in grasshopper and crayfish. Development 107:201–212
Petschek JP, Perrimon N, Mahowald AP (1987) Region specific defects in l(1) giant embryos of Drosophila melanogaster. Dev Biol 119:175–189
Sander K (1988) Studies in insect segmentation: from teratology to phenogenetics. Development 104 [Suppl]:111–121
Schnetter M (1935) Morphologische Untersuchungen über das Differenzierungszentrum in der Embryonalentwicklung der Honigbiene. Z Morphol Ökol Tiere 29:114–195
Tazima Y (1964) The genetics of the silkworm. Logos, London
Turner FR, Mahowald AP (1977) Scanning electron microscopy of Drosophila melanogaster. II. Gastrulation and segmentation. Dev Biol 57:403–416
Turner FR, Mahowald AP (1979) Scanning electron microscopy of Drosophila melanogaster. III. Formation of the head and caudal segments. Dev Biol 68:96–109
Walldorf U, Fleig R, Gehring WJ (1989) Comparison of homeo box containing genes of the honeybee and Drosophila. Proc Natl Acad Sci USA 86:9971–9975
Weir MP, Kornberg T (1985) Pattern of engrailed and fushi tarazu transcripts reveal novel intermediate stages in Drosophila segmentation. Nature 318:433–439
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fleig, R. Engrailed expression and body segmentation in the honeybee Apis mellifera . Roux's Arch Dev Biol 198, 467–473 (1990). https://doi.org/10.1007/BF00399057
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00399057