Summary
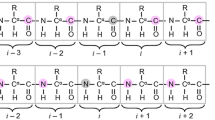
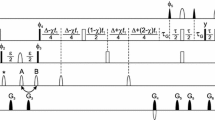
The backbone 1H and 15N resonances of the N-terminal SH3 domain of the Drosophila signaling adapter protein, drk, have been assigned. This domain is in slow exchange on the NMR timescale between folded and predominantly unfolded states. Data were collected on both states simultaneously, on samples of the SH3 in near physiological buffer exhibiting an approximately 1:1 ratio of the two states. NMR methods which exploit the chemical shift dispersion of the 15N resonances of unfolded states and pulsed field gradient water suppression approaches for avoiding saturation and dephasing of amide protons which rapidly exchange with solvent were utilized for the assignment.
Similar content being viewed by others
Abbreviations
- 2D, 3D:
-
two-, three-dimensional
- drkN SH3:
-
N-terminal SH3 domain of Drosophila drk
- HSQC:
-
heteronuclear single-quantum spectroscopy
- NOE:
-
nuclear Overhauser enhancement
- SH3:
-
Src homology domain 3
- TOCSY:
-
total correlation spectroscopy
References
AlexandrescuA. T., AbeygunawardanaC. and ShortleD. (1994) Biochemistry, 33, 1063–1072.
ArakawaT. and TimasheffS.N. (1985) Methods Enzymol., 114, 49–77.
BaxA. and PochapskyS. (1992) J. Magn. Reson., 99, 638–643.
BookerG. W., BreezeA.L., DowningA.K., PanayotouG., GoutI., WaterfieldM.D. and CampbellI.D. (1992) Nature, 358, 684–687.
CavanaghJ. and RanceM. (1992) J. Magn. Reson., 96, 670–678.
CicchettiP., MayerB.J., ThielG. and BaltimoreD. (1992) Science, 257, 803–806.
DelaglioF., NMRPipe Software System, National Institutes of Health, Bethesda, MD, 1993.
EvansP.A., ToppingD.D., WoolfsonD.N. and DobsonC.M. (1991) Proteins, 9, 248–266.
GarrettD.S., PowersR., GronenbornA.M. and CloreG.M. (1991) J. Magn. Reson., 95, 214–220.
GrzesiekS. and BaxA. (1993) J. Am. Chem. Soc., 115, 12593–12594.
KayL.E., KeiferP. and SaarinernT. (1992) J. Am. Chem. Soc., 114, 10663–10665.
KayL.E., XuG.Y. and YamazakiT. (1994) J. Magn. Reson. Ser. A, 109, 129–133.
KhodaD., HatanakaH., OdakaM., MandiyanV., UllrichA., SchlessingerJ. and InagakiF. (1993) Cell, 72, 953–960.
KochC.A., AndersonD., MoranM.F., EllisC. and PawsonT. (1991) Science, 252, 668–674.
KoyamaS., YuH., DalgarnoD.C., ShinT.B., ZydowdkyL.D. and SchreiberS.L. (1993) Cell, 72, 945–952.
LoganT.M., OlejnickzakE.T., XuR.X. and FesikS.W. (1993) J. Biomol. NMR, 3, 225–231.
LoganT.M., ThériaultY. and FesikS.W. (1994) J. Mol. Biol., 236, 637–648.
LumbK. J. and KimP.S. (1994) J. Mol. Biol., 236, 412–420.
MarionD., IkuraM., TschudinR. and BaxA. (1989) J. Magn. Reson., 85, 393–399.
McCoyM.A. and MuellerL. (1992) J. Am. Chem. Soc., 114, 2108–2110.
MuellerL., Campbell-BurkeS. and DomailleP. (1992) J. Magn. Reson., 96, 408–415.
MuhandiramD.R. and KayL.E. (1994) J. Magn. Reson. Ser. B. 103, 203–216.
MusacchioA., GibsonT., LehtoV.-P. and SarasteM. (1992a) FEBS Lett., 307, 55–61.
MusacchioA., NobleM., PaupitR., WierengaR. and SarasteM. (1992b) Nature, 359, 851–855.
NeriD., BilleterM., WiderG. and WüthrichK. (1992) Science, 257, 1559–1563.
NobleM.E.M., MusacchioA., SarasteM., CourtneidgeS.A. and WierengaR.K. (1993) EMBO J., 12, 2617–2624.
OlivierJ.P., RaabeT., HenkemeyerM., DicksonB., MbamaluG., MargolisB., SchlessingerJ., HafenE. and PawsonT. (1993) Cell, 73, 179–191.
PalmerA.G., CavanaghJ., WrightP.E. and RanceM. (1991) J. Magn. Reson., 93, 151–170.
PawsonT. and GishG.D. (1992) Cell, 71, 359–362.
SchleucherJ., SattlerM. and GriesingerC. (1993) Angew. Chem., 114, 1518–1521.
ShakaA.J., KeelerJ., FrenkielT. and FreemanR. (1983) J. Magn. Reson., 52, 335–338.
SimonM.A., DodsonG.S. and RubinG.M. (1993) Cell, 73, 169–177.
StatesD.J., HaberkornR. and RubenD.J. (1982) J. Magn. Reson., 48, 286–292.
YangY.S., GarbayC., DuchesneM., CornilleF., JullianN., FromageN., TocqueB. and RoquesB.P. (1994) EMBO J., 13, 1270–1279.
YuH., RosenM.K., ShinT.B., Seidel-DugganC., BruggeJ.S. and SchreiberS.L. (1992) Science, 258, 1665–1668.
YuH., ChenJ.K., FengS., DalgarnoD.C., BrauerA.W. and SchreiberS.L. (1994) Cell, 76, 933–945.
Zhang, O. and Forman-Kay, J.D. (1994) Biochemistry, submitted for publication.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, O., Kay, L.E., Olivier, J.P. et al. Backbone 1H and 15N resonance assignments of the N-terminal SH3 domain of drk in folded and unfolded states using enhanced-sensitivity pulsed field gradient NMR techniques. J Biomol NMR 4, 845–858 (1994). https://doi.org/10.1007/BF00398413
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00398413