Abstract
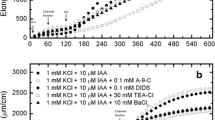
We have compared the effects of the auxin, indole-3-acetic acid (IAA) with that of other weak acids on the plasma-membrane potential of oat (Avena sativa L.) coleoptile cells. Cells treated with 1 μM IAA at pH 6 depolarize 20–25 mV in 10–12 min, but they then repolarize, until by 20–25 min their potentials are about 25 mV more negative than the initial value. Similar concentrations of benzoic and butyric acids cause the initial depolarization, but not the subsequent hyperpolarization. The hyperpolarization is therefore specific to IAA. All the weak acids, including IAA, evoke a rapid hyperpolarization when their concentrations are raised to 10 mM. This result indicates that at high concentrations, the uptake of undissociated weak acids activates electrogenic proton pumping, most likely by lowering cytoplasmic pH. In contrast, the hyperpolarization observed with concentrations of IAA four orders of magnitude lower appears to be a specific hormonal effect. This specific, auxin-induced hyperpolarization occurs at the same time as the initiation of net proton secretion and supports the hypothesis that auxin initiates extension growth by increasing proton pumping.
Similar content being viewed by others
Abbreviations
- FC:
-
fusicoccin
- IAA:
-
indole-3-acetic acid
References
Barkley, G.M., Evans, M.L. (1970) Timing of the auxin response in etiolated pea stem sections. Plant Physiol. 45, 143–147
Bates, G.W., Goldsmith, M.H.M., Goldsmith, T.H. (1982a) Separation of tonoplast and plasma membrane potential and resistance in cells of oat coleoptiles. J. Membr. Biol. 66, 15–23
Bates, G.W., Goldsmith, M.H.M., Goldsmith, T.H. (1982b) Origins and measurement of the membrane potentials in Avena coleoptiles. In: Plasmalemma and tonoplast: their functions in the plant cell. Proc. of Int. Workshop on Plasmalemma and Tonoplast of Plant Cells, pp. 241–246, Marmé, D., Marrè, E., Hertel, R., eds. Elsevier Biomedical, Amsterdam New York Oxford
Collander, R. (1959) Cell membranes: their resistance to penetration and their capacity for transport. In: Plant physiology — A treatise, vol. 2, pp. 3–102, Steward, F.C., ed. Academic Press, New York London
Cleland, R.E. (1976a) Kinetics of hormone-induced H+ excretion. Plant Physiol. 58, 210–213
Cleland, R.E. (1976b) Fusicoccin-induced growth and hydrogen ion excretion of Avena coleoptiles: relation to auxin responses. Planta 128, 201–206
Cleland, R.E. (1982) The mechanism of auxin-induced proton efflux. In. Plant growth substances 1982, pp. 22–31, Wareing, P.F., ed. Academic Press, New York London
Cleland, R.E., Prins, H.B.A., Harper, J.R., Higinbotham, N. (1977) Rapid hormone-induced hyperpolarization of the oat coleoptile potential. Plant Physiol. 59, 395–397
Cross, J.W., Briggs, W.R., Dohrmann, U.C., Ray, P.M. (1978) Auxin receptors of maize coleoptiles do not have ATPase activity. Plant Physiol. 61, 581–584
Davies, P.J., Rubery, P.H. (1978) Components of auxin transport in stem segments of Pisum sativum L. Planta 142, 211–219
Dohrmann, U., Hertel, R., Pesci, P., Cocucci, S.M., Marrè, E., Randazzo, G., Ballio, A. (1977) Localization of ‘in vitro’ binding of the fungal toxin fusicoccin to plasma membrane rich fractions from corn coleoptiles. Plant Sci. Lett. 9, 291–299
Etherton, B. (1970) Effect of indole-3-acetic acid on membrane potential of oat coleoptile cells. Plant Physiol. 45, 527–528
Felle, H. (1982) Effects of fusicoccin upon membrane potential, resistance and current-voltage characteristics in root hairs in Sinapis alba. Plant Sci. Lett. 25, 219–225
Glass, A.D.M., Dunlop, J. (1974) Influence of phenolic acids on ion uptake. Plant Physiol. 54, 855–858
Goldsmith, M.H.M. (1977) The polar transport of auxin. Annu. Rev. Plant Physiol. 28, 439–478
Goldsmith, M.H.M., Fernández, H., Goldsmith, T.H. (1972) Electrical properties of parenchymal cell membranes in the oat coleoptile. Planta 102, 302–323
Göring, H., Polevoy, V.V., Stahlberg, R., Stumpe, G. (1979) Depolarization of transmembrane potential of corn and wheat coleoptiles under reduced water potential and after IAA application. Plant Cell Physiol. 20, 649–656
Haschke, H.P., Lüttge, U. (1977) Action of auxin on CO2 dark fixation in Avena coleoptile segments as related to elongation growth. Plant Sci. Lett. 8, 53–58
Helgerson, S.L., Cramer, W.A., Morrè, D.J. (1976) Evidence for an increase in microviscosity of plasma membranes from soybean hypocotyls induced by the plant hormone indole-3-acetic acid. Plant Physiol. 58, 548–551
Jackson, P.C., Taylor, J.M. (1970) Effects of organic acids on ion uptake and retention in barly roots. Plant Physiol. 46, 538–542
Jacobs, M., Ray, P.M. (1976) Rapid auxin-induced decrease in free space pH and its relationship to auxin-induced growth in maize and pea. Plant Physiol. 58, 203–209
Loros, J., Taiz, L. (1982) Auxin increases the water permeability of Allium cells. Plant Sci. Lett. 26, 93–102
Lüttge, U., Higinbotham, N., Pallaghy, C.K. (1972) Electrochemical evidence of specific action of indole acetic acid on membranes of Mnium leaves. Z. Naturforsch. Teil B 27, 1239–1242
Marrè, E. (1977) Effects of fusicoccin and hormones on plant cell membrane activities: observations and hypotheses. In: Regulation of cell membrane activities in plants, pp. 185–202, Marrè, E., ed. Elsevier/North-Holland, Amsterdam
Marrè, E. (1979) Fusicoccin a tool in plant physiology. Annu. Rev. Plant Physiol. 30, 273–288
Marrè, E., Lado, P., Ferroni, A., Ballarin Denti, A. (1974) Transmembrane potential increase induced by IAA, benzyladenine, and fusicoccin. Correlation with proton extrusion and cell enlargement. Plant Sci. Lett. 2, 257–265
Marrè, M.T., Romani, G., Cocucci, M., Marrè, E. (1982) Internal pH and transmembrane potential as regulators of the activity of the proton pump of higher plants. International Workshop on Membranes and Transport in Biosystems, pp. 111–114, Laterza, Bari, Italy
Mizuno, A., Katuo, K., Okamoto, H. (1980) Structure and function of the elongation sink in the stems of higher plants. I. Effects of anoxia and IAA on the growth rate and the spatially separate electrogenic ion pumps. Plant Cell Physiol. 21, 395–403
Morath, M., Hertel, R. (1978) Lateral electric potential following asymmetric auxin application to amize coleoptiles. Planta 140, 31–35
Morrè, D.J., Bracker, C.E. (1976) Ultrastructural alteration of plant plasma membranes induced by auxin and calcium ions. Plant Physiol. 58, 544–547
Nelles, A. (1977) Short term effects of plant hormones. Planta 147, 293–298
Poole, R.J. (1978) Energy coupling for membrane transport. Annu. Rev. Plant Physiol. 29, 437–460
Rayle, D.L., Evans, M.L., Hertel, R. (1970) Action of auxin on cell elongation. Proc. Natl. Acad. Sci. USA 65, 184–191
Rayle, D.L., Cleland, R.E. (1977) Control of plant cell enlargement by hydrogen ions. Curr. Top. Dev. Biol. 11, 187–211
Rubinstein, R., Cleland, R.E. (1981) Responses of Avena coleoptiles to suboptimal fusicoccin: kinetics and comparisons with indoleacetic acid. Plant Physiol. 68, 543–547
Rubery, P.H., Sheldrake, A.R. (1974) Carrier-mediated auxin transport. Planta 118, 101–121
Sanders, D., Hansen, U.P., Slayman, C.L. (1981) Role of plasma membrane proton pump in pH regulation in non-animal cells. Proc. Natl. Acad. Sci. USA 78, 5903–5907
Smith, F.A., Raven, J.A. (1976) H+ transport and regulation of cell pH. In: Encyclopedia of plant physiology, N.S., vol. 2: Transport in plants IIA: Cells, pp. 317–346, Lüttge, U., Pitman, M., eds. Springer, Berlin Heidelberg New York
Spanswick, R.M. (1981) Electrogenic ion pumps. Annu. Rev. Plant Physiol. 32, 267–289
Stahlberg, R., Polevoy, V.V. (1979) Nature of the rhythmic oscillations of the membrane potential in corn coleoptile cells, [in Russian]. Dokl. Bot. Sci. 247, 74–76
Sussman, M.R., Goldsmith, M.H.M. (1981) Auxin uptake and action of N-1-naphthylphthalamic acid in corn coleoptiles. Planta 151, 15–25
Sweeney, B.M., Thimann, K.V. (1937) The effects of auxins on protoplasmic streaming, II. J. Gen. Physiol. 25, 439–461
Vian, B., Mosiniak, M., Roland, J.-Cl. (1976) Alterations ultrastructurales du plasmalemme de Phaeolus aureus induites par l'auxine sur cellules entières et membranes isolées. Ann. Sci. Nat. Bot. Sér. 12, 17, 105–118
Ullrich, C.H. (1978) Continuous measurement of initial curvature of maize coleoptiles induced by lateral auxin application. Planta 140, 201–211
Yamaki, T. (1954) Effect of indoleacetic acid upon oxygen uptake, carbon dioxide fixation and elongation of Avena coleoptile cylinders in darkness. Sci. Pap. Coll. Gen. Educ. Univ. Tokyo 4, 127–154
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bates, G.W., Goldsmith, M.H.M. Rapid response of the plasma-membrane potential in oat coleoptiles to auxin and other weak acids. Planta 159, 231–237 (1983). https://doi.org/10.1007/BF00397530
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00397530