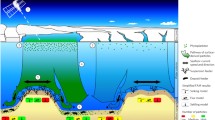
Abstract
Energy flow through continental shelf food webs was examined using a simulation model. The model structure expands the two traditional marine food chains of phytoplankton-zooplankton-pelagic fish and benthos-demersal fish into a complex web which includes detritus, dissolved organic matter (DOM), bacteria, protozoa, and mucus net feeders. Simulation of energy flux for different shelf systems using the expanded web revealed that heterotrophic microorganisms and their predators account for a significant component of the energy flux in the continental shelf ecosystem. Contrary to previous models, where all phytoplankton were considered to be grazed by zooplankton, our simulation results indicate that only slightly more than 50% of the annual net primary production is grazed. A substantial quantity of the phytoplankton production directly becomes detritus. Bacteria mineralize detritus and DOM produced by phytoplankton and other components of the food web, converting these to biomass with high efficiency. Consequently, the model predicts that planktonic bacterial production is equivalent to zooplankton production. Exclusion of the bacteria requires the assumption that all DOM is either exported from the system or consumed by another component of the food web. Neither of these assumptions can be supported by present knowledge of the dynamics of DOM in the sea. Model simulations were also employed to test the hypothesis that production exceeds consumption on continental shelves, resulting in exports of 50% of the annual primary production. Simulations of shelves with high rates of primary production resulted in a particulate export of 27% and realistic estimates of secondary production. Results of other simulations suggest that shelves with lower primary production cannot export production and still maintain the macrobenthos and their predators. General properties about continental shelves can also be inferred from the model. From simulations of shelves of differing primary production, nanoplankton are predicted to account for a greater proportion of the primary production in nutrient limited systems. Benthic production appears to be related to both the quantity of primary production and the sinking rates of the phytoplankton. The model indicates that zooplankton fecal inputs to the shelf benthos are only a small portion of the total detrital flux, leading to the prediction that fecal pellets are of little significance in determining benthic production. Finally, the model generates production efficiencies that are highly variable depending on the type of system and kind of populations involved. We argue that the assumed ecological efficiency of 10% should be abandoned for continental shelves and other ecosystems.
Similar content being viewed by others
Literature cited
Atkinson, L. P.: Modes of Gulf Stream intrusion into the South Atlantic Bight shelf waters. Geophys. Res. Lett. 7, 583–586 (1977)
Azam, F. and R. E. Hodson: Size distribution and activity of marine microheterotrophs. Limnol. Oceanogr. 22, 492–501 (1977)
Azam, F., T. Fenchel, J. G. Field, J. S. Gray, L. A. Meyer-Reil and F. Thingstad. The ecological role of water column microbes in the sea. Mar. Ecol. Prog. Ser. 10, 257–263 (1983)
Banse, K.: Rates of growth, respiration, and photosynthesis of unicellular algae as related to cell size — a review. J. Phycol. 12, 135–140 (1976)
Banse, K.: On weight dependence of net growth efficiency and specific respiration rates among field populations. Oecologia 38, 111–126 (1979)
Banse, K. and S. Mosher. Adult body mass and annual production/biomass relationships of field populations. Ecol. Monogr. 50, 355–379 (1980)
Barber, R. T. and R. L. Smith: Coastal upwelling ecosystems. In: Analysis of marine ecosystems, pp 31–68. Ed. by A. R. Longhurst. New York: Academic Press 1981
Birkett, L.: Experimental determination of food conversion and its application to ecology. In: Marine food chains, pp 261–264. Ed. by J. H. Steele. Berkley. University of California Press 1970
Blanton, J. O., L. P. Atkinson, L. J. Pietrafesa and T. N. Lee: The intrusion of Gulf Stream water across the continental shelf due to topographically-induced upwelling. Deep-Sea Res. 28, 393–405 (1981)
Buchanan, J. B. and R. M. Warwick: An estimate of benthicmicrofaunal production in the offshore mud of the Northumberland coast. J. mar. biol. Assoc. U.K. 54, 197–222 (1974)
Conover, R. J.: Transformation of organic matter. In: Marine ecology, Vol. 4, pp 221–500. Ed. by O. Kinne. New York: Wiley 1978
Cooney, R. J. and K. O. Coyle: Trophic implications of crossshelf copepod distributions in the southeastern Bering Sea. Mar. Biol. 70, 187–196 (1982)
Copping, A. E. and C. J. Lorenzen: Carbon budget of a marine phytoplankton-herbivore system with carbon-14 as a tracer. Limnol. Oceanogr. 25, 873–882 (1980)
Cosper, T. C. and M. R. Reeve. Digestive efficiency of the chaetognath Sagitta hispida. J. exp. mar. Biol. Ecol. 17, 33–38 (1975)
Coull, B. C. and W. B. Vernberg: Harpacticoid copepod respiration: Enhydrosoma propinguum and Longipedia helgolandica. Mar. Biol. 5, 341–344 (1970)
Cuhel, R. L., H. W. Jannasch, C. D. Taylor and D. R. S. Lean: Microbial growth and macromolecular synthesis in the northwestern Atlantic. Limnol. Oceanogr. 28, 1–18 (1983)
Dagg, M. J. and J. T. Turner: The impact of copepod grazing on the phytoplankton of Georges Bank and the New York Bight. Can. J. Fish. aquat. Sci. 39, 979–990 (1982)
Dagg, M. J., J. Vidal, T. E. Whitledge, R. L. Iverson and J. J. Goering: The feeding, respiration, and excretion of zooplankton in the Bering Sea during a spring bloom. Deep-Sea Res. 29, 45–63 (1982)
Deibel, D.: Laboratory determined mortality, fecundity and growth rates of Thalia democratica Forskal and Dolioletta gegenbauri Uljanin (Tunicata, Thaliacea). J. Plank. Res. 4, 143–153 (1982a)
Deibel, D.: Laboratory measured grazing and ingestion rates of the salp, Thalia democratica Forskal, and the doliolid Dolioletta gegenbauri Jljanin (Tunicata, Thaliacea). J. Plank. Res. 4, 189–201 (1982b)
Ducklow, H. W.: Production and fate of bacteria in the ocean. BioScience 33, 494–501 (1983)
Eppley, R. W., J. N. Rogers and J. J. McCarthy: Half saturation constants for uptake of nitrate and ammonium by marine phytoplankton. Limnol. Oceanogr. 14, 912–920 (1969)
Fenchel, T.: Ecology of heterotrophic microflagellates. II. Bioenergetics and growth. Mar. Ecol. Prog. Ser. 8, 225–231 (1982)
Flint, R. W. and N. N. Rabalais: Environmental studies of a marine ecosystem. Austin, Texas: Univ. of Texas Press 1981
Frost, B. W.: Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnol. Oceanogr. 17, 805–815 (1972)
Fuhrman, J. A. and F. Azam: Bacterioplankton secondary production estimates for coastal waters of British Columbia, Antarctica, and California. Appl. environ. Microbiol. 39, 1085–1095 (1980)
Gerlach, S. A.: Food-chain relationships in subtidal silty sand marine sediments and the role of meiofauna in stimulating bacterial productivity. Oecologia 33, 55–69 (1978)
Haines, E. B. and W. M. Dunstan: The distribution and relation of particulate organic material and primary productivity in the Georgia Bight. Estuar. coast. mar. Sci. 3, 431–441 (1975)
Hargrave, B. T.: The utilization of benthic microflora by Hyallela azteca (Amphipoda). J. Anim. Ecol. 39, 427–437 (1970)
Hargrave, B. T.: Factors affecting the flux of organic matter to sediments in a marine bay. In: Marine benthic dynamics, pp 219–222. Ed. by K. R. Tenore and B. C. Coull. Columbia: University of South Carolina Press 1980
Heinbokel, J. F.: Studies on the functional role of Tintinnids in the Southern California Bight. I. Grazing and growth rates in laboratory cultures. Mar. Biol. 47, 177–189 (1978)
Hobbie, J. E. and C. C. Crawford: Respiration corrections for bacterial uptake of dissolved organic compounds in natural waters. Limnol. Oceanogr. 14, 528–532 (1969)
Hodson, R. E., A. E. Maccubbin and L. R. Pomeroy: Dissolved adenosine triphosphate utilization by free living and attached bacterioplankton. Mar. Biol. 64, 43–51 (1981)
Hofmann, E. E., J. M. Klinck and G.-A. Paffenhöfer: Concentrations and vertical fluxes of zooplankton fecal pellets on a continental shelf. Mar. Biol. 61, 327–335 (1981)
Johannes, R. E. and M. Satomi: Measuring organic matter retained by aquatic invertebrates. J. Fish. Res. Bd Can. 24, 2467–2471 (1967)
Karl, D. M.: Measurements of microbial activity and growth in the ocean by rates of stable ribonucleic acid synthesis. Appl. environ. Microbiol. 33, 777–783 (1979)
Kay, D. G. and A. E. Brafield. The energy relations of the polychaeta Neanthes (Nereis) virens (Sars). J. Anim. Ecol. 42, 673–692 (1973)
Kofoed, L. H.: The feeding biology of Hydrobia ventrosa Montagu. II. Allocation of the components of the carbon budget and the significance of the secretion of dissolved organic material. J. exp. mar. Biol. Ecol. 19, 243–256 (1975)
Lampert, W.: Release of dissolved organic carbon by grazing zooplankton. Limnol. Oceanogr. 23, 831–834 (1978)
Lasker, R.: Utilization of zooplankton energy by a Pacific sardine population in the California current. In: Marine food chains, pp 265–284. Ed. by J. H. Steele. Berkely: Univ. of California Press 1970
Lee, C. and J. L. Bada: Dissolved amino acids in the equatorial Pacific, the Sargasso Sea, and Biscayne Bay. Limnol. Oceanogr. 22, 502–510 (1977)
Lee, J. J., J. H. Tietjen, N. M. Saks, G. G. Ross, H. Rubin and W. A. Muller: Educing and modeling the functional relationships within sublittoral salt march aufwuchs communities — inside one of the black boxes. In: Estuarine research, Vol. 1, pp 710–734. Ed. by L. E. Cronin. New York: Academic Press 1975
Madin, L. P.: Field observations on the feeding behavior of salps. (Tunicata, Thaliacea). Mar. Biol. 25, 143–147 (1974)
Mague, T. H., E. Friberg, D. J. Hughes and I. Morris: Extracellular release of carbon by marine phytoplankton; a physiological approach. Limnol. Oceanogr. 25, 262–279 (1980)
Malone, T. C.: Size fractioned primary productivity of marine phytoplankton. In: Primary productivity in the sea, pp 301–319. Ed. by P. G. Falkowski. New York: Plenum 1980
McAllister, C. D., N. Shah and J. D. H. Strickland: Marine phytoplankton photosynthesis as a function of light intensity: a comparison of methods. J. Fish. Res. Bd Can. 21, 159–181 (1964)
Mills, E. L.: The structure and dynamics of shelf and slope ecosystems off the North East coast of North America. In: Marine benthic dynamics, pp 25–47. Ed. by K. R. Tenore and B. C. Coull. Columbia: University of South Carolina Press 1980
Mullin, M. M.: Production of zooplankton in the ocean: The present status and problems. Oceanogr. mar. Biol. Ann. Rev. 7, 293–314 (1969)
Mullin, M. M. and E. A. Brooks: Growth and metabolism of two marine planktonic copepods as influenced by temperature and type of food. In: Marine food chains, pp 74–95. Ed. by J. H. Steele. Berkley: University of California Press 1970
Nichols, F. H.: Dynamics and energetics of three deposit-feeding benthic invertebrate populations in Pudget Sound, Washington. Ecol. Monogr. 45, 57–82 (1975)
Paffenhöfer, G.-A. and S. C. Knowles: Feeding of marine planktonic copepods on mixed phytoplankton. Mar. Biol. 48, 143–152 (1978)
Paffenhöfer, G.-A. and S. C. Knowles: Ecological implications of fecal pellet size, production and consumption by copepods. J. mar. Res. 37, 35–49 (1979)
Parsons, T. R. and R. J. LeBrasseur: The availability of food to different trophic levels in a marine food chain. In: Marine food chains, pp 325–343. Ed. by J. H. Steele. Berkley: University of California Press 1970
Parsons, T. R., M. Takahashi and B. Hargrave: Biological oceanographic processes, 332 pp. New York: Pergamon 1977
Payne, W. J. and W. J. Wiebe: Growth yields and efficiency in chemosynthetic microorganisms. Ann. Rev. Microbiol. 32, 155–183 (1978)
Peters, R.: Ecological implications of body size, 329 pp. Cambridge: Cambridge University Press 1983
Platt, T. and B. Irwin: Caloric content of phytoplankton. Limnol. Oceanogr. 18, 306–310 (1973)
Pomeroy, L. R.: The ocean's food web, a changing paradigm. BioScience 24, 499–504 (1974)
Pomeroy, L. R.: Secondary production mechanisms of continental shelf communities. In: Ecological processes in coastal and marine ecosystems, pp 163–186. Ed. by R. J. Livingstone. New York: Plenum 1979
Pomeroy, L. R.: Significance of microorganisms in carbon and energy flow in marine ecosystems. In: Perspectives in microbial ecology. Ed. by M. J. Klug and C. A. Reddy. Am. Soc. Microbiol.: Washington 1984
Reeve, M. R.: Comparative experimental studies on the feeding of chaetognaths and ctenophores. J. Plank. Res. 2, 381–393 (1980)
Riley, G. A.: Factors controlling phytoplankton populations on Georges Bank. J. mar. Res. 6, 54–73 (1946)
Riley, G. A., H. Stommel and D. A. Bumpus: Quantitative ecology of the plankton of the western North Atlantic. Bull. Bingham. Oceanogr. Coll. 12, 1–169 (1949)
Rubin, H. A. and J. J. Lee: Informational energy flows as an aspect of the ecological efficiency of marine ciliates. J. theor. Biol. 62, 69–91 (1976)
Sameoto, D. D.: Yearly respiration rate and estimated energy budget for Sagitta elegans. J. Fish. Res. Bd Can. 29, 987–996 (1972)
Slobodkin, L. B.: Growth and regulation of animal populations, 184 pp. New York: Holt, Rinehart, and Winston 1961
Small, L. F., H. Curl and W. A. Glooschenko. Estimates of primary production off Oregon using an improved chlorophyll light technique. J. Fish. Res. Bd Can. 29, 1261–1267 (1972)
Smayda, T. J.: The suspension and sinking of phytoplankton in the sea. Oceanogr. mar. Biol. Ann. Rev. 8, 353–414 (1970)
Smith, P. E. and R. W. Eppley: Primary production and the anchovy population in the Southern California Bight: comparison of time series. Limnol. Oceanogr. 27, 1–17 (1982)
Steele, J. H.: The structure of marine ecosystems, 128 pp. Cambridge: Harvard University Press 1974
Steele, J. H. and B. W. Frost: The structure of plankton communities. Philos. Trans. R. Soc. Lond. Ser. B. 280, 485–534 (1977)
Steele, J. H. and E. W. Henderson. A simple plankton model. Am. Nat. 117, 676–691 (1981)
Tenore, K. R., L. Cammen, S. E. G. Findlay, and N. Phillips: Perspectives of research on detritus: do factors controlling the vailability of detritus to macro-consumers depend on its source. J. mar. Res. 40, 473–490 (1982)
Thomas, J. P.: The influence of the Altamaha River on primary production beyond the mouth of the river, 60 pp. M. S. thesis. University of Georgia, Athens 1966
Vaccaro, R. F., S. E. Hicks, H. W. Jannasch and F. G. Carey: The occurrence and role of glucose in seawater. Limnol. Oceanogr. 13, 356–360 (1968)
Walsh, J. J.: A spatial simulation model of the Peru upwelling ecosystem. Deep-Sea Res. 22, 201–236 (1975)
Walsh, J. J.: A carbon budget for overfishing off Peru. Nature, Lond. 290, 300–304 (1981a)
Walsh, J. J.: Shelf-sea ecosystems. In: Analysis of marine ecosystems, pp 159–198. Ed. by A. R. Longhurst. New York: Academic Press 1981 b
Walsh, J. J., T. E. Whitledge, F. W. Barnevik, C. D. Winick, S. O. Howe, W. E. Esaias and J. T. Scott: Wind events and food chain dynamics within the New York Bight. Limnol. Oceanogr. 23, 659–683 (1978)
Walsh, J. J., G. T. Rowe, R. L. Iverson and C. P. McRoy: Biological export of shelf carbon is a sink of the global CO2 cycle. Nature, Lond. 291, 196–201 (1981)
Wiegert, R. G.: Mathematical representation of ecological interactions. In: Ecosystem analysis and prediction. Proceedings of the SIAM, SIMS conference, Alta, Utah pp 43–53. Ed. by S. A. Levin. Philadelphia: Philadelphia Society of Industrial and Applied Mathematics 1975
Wiegert, R. G.: Population models: experimental tools for analysis of ecosystems. In: Proceedings of colloquium on analysis of ecosystems, pp 239–279. Ed. by D. J. Horn, R. Mitchell and G. R. Stairs. Columbus: Ohio State University Press 1979
Wiegert, R. G., R. R. Christian and R. L. Wetzel: A model view of the marsh. In: Ecology of a salt marsh, pp 183–218. Ed. by L. R. Pomeroy and R. G. Wiegert. New York: Springer-Verlag 1981
Williams, P. J. leB.: Heterotrophic utilization of dissolved organic compounds in the sea. I. Size distribution of population and relationship between respiration and incorporation of growth substrates. J. mar. biol. Assoc. U.K. 50, 859–870 (1970)
Williams, P. J. leB.: Incorporation of microheterotrophic processes into the classical paradigm of the planktonic food web. Kieler Meeresforsch., Sonderh. 5, 1–28 (1981)
Wood, J. D., Nitrogen excretion in some marine teleosts. Can. J. Biochem. Physiol. 36, 1237–1242 (1958)
Wright, R. T.: Measurement and significance of specific activity in the heterotrophic bacteria of natural waters. Appl. environ. Microbiol. 36, 297–305 (1978)
Wright, R. T. and J. E. Hobbie: Use of glucose and acetate by bacteria and algae in aquatic systems. Ecology. 47, 447–464 (1966)
Yingst, J. Y.: The utilization of organic matter in shallow marine sediments by an epibenthic deposit-feeding holothurian. J. exp. mar. Biol. Ecol. 23, 55–69 (1976)
Yoder, J. A., L. P. Atkinson, S. S. Bishop, E. E. Hofmann and T. N. Lee: Effect of upwelling on phytoplankton productivity on the outer southeastern U.S. continental shelf. Limnol. Oceanogr. 26, 1103–1110 (1981)
Author information
Authors and Affiliations
Additional information
Communicated by R. W. Doyle, Halifax
Rights and permissions
About this article
Cite this article
Pace, M.L., Glasser, J.E. & Pomeroy, L.R. A simulation analysis of continental shelf food webs. Mar. Biol. 82, 47–63 (1984). https://doi.org/10.1007/BF00392763
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00392763