Abstract
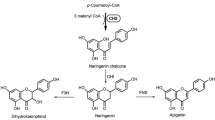
Chalcone synthase activity was demonstrated in enzyme preparations from flowers of defined genotypes of Dianthus caryophyllus L. (carnation). In the absence of chalcone isomerase activity, which could be completely excluded by genetic methods, the first product formed from malonyl-CoA and 4-coumaroyl-CoA proved to be naringenin chalcone, followed by formation of naringenin as a result of chemical cyclization. In the presence of chalcone isomerase activity, however, naringenin was the only product of the synthase reaction. In vitro, both 4-coumaryl-CoA and caffeoyl-CoA were found to be used as substrates for the condensation reaction with respective pH optima of 8.0 and 7.0. The results of chemogenetic and enzymatic studies, however, showed that in vivo only 4-coumaroyl-CoA serves as substrate for the formation of the flavonoid skeleton. In confirmation of these results, an NADPH-dependent microsomal 3′-hydroxylase activity could be demonstrated, catalyzing hydroxylation of naringenin and dihydrokaempferol in 3′-position. Furthermore, a strict correlation was found between 3′-hydroxylase activity and the gene r which is known to control the formation of 3′, 4′-hydroxylated flavonoid compounds.
Similar content being viewed by others
References
Barton, G.M. (1968) Detection of 3-hydroxyflavanones on papergrams and thin-layer plates. J. Chromatogr. 34, 562
Bradord, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal. Biochem. 72, 248–254
Eigen, E., Blitz, M., Ginsberg, E. (1957) The detection of some naturally occuring flavanone compounds on paper chromatograms. Arch. Biochem. Biophys. 68, 501
Forkmann, G., Dangelmayr, B. (1980) Genetic control of chalcone isomerase activity in flowers of Dianthus caryophyllus. Biochem. Genet. 18, 519–527
Forkmann, G., Heller, W., Grisebach, H. (1980) Anthocyanin biosynthesis in flowers of Matthiola incana. Flavanone 3-and flavonoid 3′-hydroxylases. Z. Naturforsch. 35c, 691–695
Forkmann, G., Kuhn, B. (1979) Genetic control of chalcone isomerase activity in anthers of Petunia hybrida. Planta 144, 189–192
Forkmann, G., Stotz, G. (1981) Genetic contron of flavanone 3-hydroxylase activity and flavonoid 3′-hydroxylase activity in Antirrhinum majus (snapdragon). Z. Naturforsch. 36c, 411–416
Geissmann, T.A., Mehlquist, G.A.L. (1947) Inheritance in the carnation, Dianthus caryophyllus. IV. The chemistry of flower colour variation. Genetics 32, 410
Heller, W., Hahlbrock, K. (1980) Highly purified flavanone synthase from parsley catalyzes the formation of naringenin chalcone. Arch. Biochem. Biophys. 200, 617–619
Kreuzaler, F., Hahlbrock, K. (1972) Enzymatic synthesis of aromatic compounds in higher plants: formation of naringenin from p-coumaroyl-CoA and malonyl-CoA. FEBS Lett. 28, 69–72
Kreuzaler, F., Hahlbrock, K. (1975) Enzymatic synthesis of an aromatic ring from acetate units. Eur. J. Biochem. 56, 205–213
Kuhn, B., Forkmann, G., Seyffert, W. (1978) Genetic control of chalcone-flavanone isomerase activity in Callistephus chinensis. Planta 138, 199–203
Moustafa, E., Wong, E. (1967) Purification and properties of chalcone flavanone isomerase from soya bean seed. Phytochemistry 6, 625–632
Saleh, N.A.M., Fritsch, M., Kreuzaler, F., Grisebach, H. (1978) Flavanone synthase from cell suspension cultures of Haplopappus gracilis and comparision with the synthase from parsley. Phytochemistry 17, 183–186
Schröder, J., Heller, W., Hahlbrock, K. (1979) Flavanone synthase: Simple and rapid assay for the key enzyme of flavonoid biosynthesis. Plant Sci. Lett. 14, 281–286
Spribille, R., Forkmann, G. (1981) Genetic control of chalcone synthase activity in flowers of Matthiola incana R. Br. Z. Naturforsch. 36c, 619–624
Spribille, R., Forkmann, G. (1982) Genetic control of chalcone synthase activity in flowers of Antirrhinum majus. Phytochemistry (in press)
Stöckigt, J., Zenk, M.M. (1975) Chemical synthesis and properties of hydroxycinnamoyl-coenzyme A derivatives. Z. Naturforsch. 30c, 352–358
Stotz, G., Forkmann, G. (1981) Oxidation of flavanones to flavones with flower extracts of Antirrhinum majus (snapdragon). Z. Naturforsch. 36c, 737–741
Sütfeld, R., Wiermann, R. (1980) Chalcone synthesis with enzyme extracts from tulip anthers tapetum using a biphasic enzyme assay. Arch. Biochem. Biophys. 201, 64–72
Sütfeld, R., Wiermann, R. (1981) Purification of chalcone synthase from tulip anthers and comparison with the synthase from Cosmos petals. Z. Naturforsch. 36c, 30–34
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spribille, R., Forkmann, G. Chalcone synthesis and hydroxylation of flavonoids in 3′-position with enzyme preparations from flowers of Dianthus caryophyllus L. (carnation). Planta 155, 176–182 (1982). https://doi.org/10.1007/BF00392549
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00392549