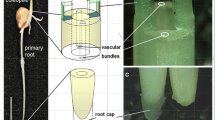
Abstract
Amyloplast sedimentation in gravistimulated maize (Zea mays L.) roots was measured using the change in angle from the center of the cell to each amyloplast as an index of sedimentation. Using tissue fixed after gravistimulation, the relationship between mean amyloplast angle and the duration of gravistimulation was found to be linear when plotted on a logarithmic time scale. Extrapolated values for the onset of angular change are 5.9 s after the start of gravistimulation for the entire population of amyloplasts and 11.8 s for lead amyloplasts. By multiplying the instantaneous angular velocity (in radians) by the cell center to amyloplast radius, it is possible to calculate the initial sedimentation velocity to be 19.1 μm min-1 at 5.9 s. During sedimentation, the mean amyloplast angles surpass the calculated cell corner angle of 123° at 2.2 min for all amyloplasts and at 19 s for lead amyloplasts near the new lower wall. Thus, substantial sedimentation occurs within the presentation time, calculated to be 4.1 min. These kinetics are consistent with several hypotheses of graviperception.
Similar content being viewed by others
Abbreviations
- tp :
-
presentation time
References
Audus, L.J. (1979) Plant geosensors. J. Exp. Bot. 30, 1051–1074
Clifford, P.E. (1979) Amyloplast movement and the geotropic response. Z. Pflanzenphysiol. 91, 69–74
Dedolph, R.R., Naqvi, S.M., Gordon, S.A. (1965) Effect of gravity compensation on the geotropic sensitivity of Avena seedlings. Plant Physiol. 40, 961–965
Griffiths, H.J.(1963) Physiological and cytological studies of the statolith apparatus in plants. Ph.D. thesis, University of London, UK
Griffiths, H.J., Audus, L.J. (1964) Organelle distribution in the statocyte cells of the root-tip of Vicia faba in relation to geotropic stimulation. New Phytol. 63, 319–333
Haberlandt, G. (1914) Physiological plant anatomy, 4th edn., transln. by M. Drummond, MacMillan, London
Hawker, L.E. (1932) A quantitative study of the geotropism of seedlings with special reference to the nature and development of their statolith apparatus. Ann. Bot. 46 121–157
Hestnes, A., Iversen, T.-H. (1978) Movement of cell organelles and the geotropic curvature in roots of Norway spruce (Picea abies) Physiol. Plant. 42, 406–414
Iversen, T.-H., Pedersen, K., Larsen, P. (1968) Movement of amyloplasts in the root cap cells of geotropically sensitive roots. Physiol. Plant. 21, 811–819
Iversen, T.-H., Larsen, P. (1973) Movement of amyloplasts in the statocytes of geotropically stimulated roots. The preinversion effect. Physiol. Plant. 28, 172–181
Johnsson, A., Pickard, B.G. (1979) The threshold stimulus for geotropism. Physiol. Plant. 45, 315–319
Larsen, P. (1962) Geotropism: An introduction Encyclopedia of plant physiology, vol. 17: Physiology of movements, pt. 2, pp. 153–199, Ruhland, W., ed. Springer, Berlin Göttinger Heidelberg
Larsen, P. (1965) Geotropic responses in roots as influenced by their orientation before and after stimulation. Physiol. Plant. 18, 747–765
Moore, R., McClelen, C.E. (1983) Ultrastructural aspects of cellular differentiation in the root cap of Zea mays. Can. J. Bot. 61, 1566–1572
Olsen, G.M., Iversen, T.-H. (1980) Ultrastructure and movements of cell structures in normal pea Pisum sativum cultivar sabel and an ageotropic mutant. Physiol. Plant. 50, 275–284
Perbal, G. (1978) Mechanism of geoperception in lentil roots. J. Exp. Bot. 29, 631–638
Pickard, B.G. (1973) Geotropic response patterns of the Avena colcoptile. I. Dependence on angle and duration of stimulation. Can. J. Bot. 51, 1003–1021
Sack, F.D., Leopold, A.C. (1985) Cytoplasmic streaming affects gravity-induced amyloplast sedimentation in maize coleoptiles. Planta 164, 56–62
Sack, F.D., Suyemoto, M.M., Leopold, A.C. (1984) Kinetics of amyloplast sedimentation in gravistimulated maize coleoptiles. Planta 161, 459–464
Sievers, A., Behrens, H.M., Buckhout, T.J., Gradmann, D. (1984) Can a Ca2+ pump in the endoplasmic reticulum of the Lepidium root be the trigger for rapid changes in membrane potential after gravistimulation? Z. Pflanzenphysiol. 114, 195–200
Spurr, A.R. (1969) A low-viscosity epoxy resin embedding medium for electron microscopy Stain Technol. 9, 43–46
Volkmann, D., Sievers, A. (1979) Graviperception in multicellular organs. Encyclopedia of plant physiology, N.S., vol 7: Physiology of movements pp. 573–600, Haupt, W., Feinleib, M., eds, Springer, Berlin Heidelberg New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sack, F.D., Suyemoto, M.M. & Leopold, A.C. Amyloplast sedimentation kinetics in gravistimulated maize roots. Planta 165, 295–300 (1985). https://doi.org/10.1007/BF00392225
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00392225