Summary
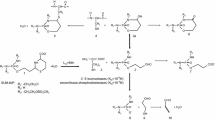
Presumably the coadministration of the uroprotector mesna in cyclophosphamide treatment does not influence the systemic activity of its activated metabolite. This was newly investigated in a mouse model. The LD50 values of i.p. administered mafosfamide, a derivative of act. CP, were increased by the simultaneous i.p. administration of mesna (mafosfamide: mesna 1: 2 on a molar weight basis) from 590 mg/kg to 750 mg/kg, and after i.v. injection of cytostatic and thiol from 505 mg/kg to 810 mg/kg. Administration of 2 × molar cysteine i.v. or i.p. to mafosfamidetreated animals was even more effective against its lethal toxicity (LD50 i.p. 1800 mg/kg and i.v. 1130 mg/kg). Bone marrow toxicity (severe leukocytopenia) was partially abolished by both thiols. Also the therapeutic efficacy of act. CP against L1210 leukemia in DBA2 mice was reduced by 50% in the presence of cysteine and of mesna. Compared with mesna the higher detoxification effect of cysteine is attributed to its longer half-life (t 1/2 20 min vs 12 min of mesna) and presumably an accumulation of cysteine in some cell systems (distribution coefficient 1.20 ml/g vs 0.68 ml/g of mesna). Nevertheless, our study clearly demonstrates a distinct systemic deactivation of act. CP by mesna, which might be of clinical relevance.
Similar content being viewed by others
Abbreviations
- act. CP:
-
activated cyclophosphamide
- SH:
-
Sulfhydryl
References
Araujo CE, Tessler J (1983) Treatment of ifosfamide-induced urothelial toxicity by oral administration of sodium 2-mercaptoethane sulphonate (mesna) to patients with inoperable lung cancer. Eur J Clin Oncol 19:195–201
Ball CR (1966) Estimation and identification of thiols in rat spleen after cysteine treatment: relevance to protection against nitrogen mustard. Biochem Pharmacol 15:319–324
Berrigan MJ, Marinello AJ, Pavelic Z, Williams CJ, Struck RF, Gurtoo HL (1982) Protective role of thiols in cyclophosphasmide induced urotoxicity and depression of hepatic drug metabolism. Cancer Res 42:3688–3695
Brade WP, Herdrich K, Varini M (1985) Ifosfamide-pharmacology, safety and therapeutic potential. Cancer Treat Rev 12:1–47
Brandt EL, Griffin AC (1951) Reduction of toxicity of nitrogen mustards by cysteine. Cancer 4:1030–1035
Brock N, Pohl J (1983) The developement of mesna for regional detoxification. Cancer Treat Rev 10: (Suppl A): 33–43
Brock N, Hohorst HJ (1977) The problem of specific and selectivity of alkylating cytostatics: studies on N-2-chloro-ethyl amidooxazaphosphorines. Z Krebsforsch 88:185–215
Brock N, Pohl J, Stekar J, Scheef W (1982) Studis on the urotoxicity of oxazaphosphorine cytostatics and its prevention. III. Profile of action of sodium 2-mercaptoethane sulfonate (mesna). Eur J Cancer Clin Oncol 18:1377–1387
Brock N, Hilgard P, Pohl J, Ormstadt K, Orrenius S (1984) Pharmacokinetics and mechanism of action of detoxifying low-molecular-weight thiols. J Cancer Res Clin Oncol 108:87–97
Connors TA (1966) Protection against the toxicity of alkylating agents by thiols: the mechanism of protection and its relevance to cancer chemotherapy. Eur J Cancer 2:293–305
Draeger U, Peter G, Hohoerst HJ (1976) Deactivation of cyclophosphamide (NSC-26271) metabolites by sulfhydryl compounds. Cancer Treat Rep 60:355–359
Grasetti DR, Murray TF Jr (1967) Determination of sulfhydryl groups with 2,2′-and 4,4′ dithiopyridine. Arch Biochem Biophys 119:41–49
Gurtoo HL, Hipkens JH, Sharma SD (1981) Role of glutathione in the metabolism dependent toxicity and chemotherapy of cyclophosphamide. Cancer Res 41:3584–3591
Hohoye PY, Duelge J, Hansen RM, Ritch PS, Anderson T (1983) Prophylaxis of ifosfamide toxicity with oral acetylcysteine. Semin Oncol 10 (Suppl 1):66–71
Kline L, Gang M, Woodman RJ, Cysyk R, Venditti JM (1973) Protection with N-acetyl-cysteine (NSL-11180) against isophosphamide (NSL-109724) toxicity and enhancement of therapeutic effect in early murine L1210 leukemia. Cancer Chemother Rep 57:299–304
Link H, Neet V, Niethammer D, Wilms K (1981) Prophylaxis of haemorrhagic cystitis due to cyclophosphamide-conditioning for bone marrow transplantation. Blut 43:329–330
Litchfield JT Jr, Wilcoxon F (1949) A simplified method of evaluating dose-effect experiments. J Pharmacol 95:99–113
Loehrer PJ, Williams SD, Einhorn LH (1983) N-acetylcysteine and ifosfamide in the treatment of unreasectable pancreatic adenocarcinoma and refractory testicular cancer. Semin Oncol 10 (Suppl 1):72–75.
Norpoth K, Müller G, Raidt H (1976) Studies on the metabolism of isophosphamide (NSC-109724) in man. Cancer Treat Rep 60:437–443
Ormstad K, Orrenius S, Lastbom T, Nehava N, Pohl J, Stekar J, Brock N (1983) Pharmacokinetics and metabolism of sodium 2-mercaptoethanesulfonate in the rat. Cancer Res 43:333–338
Pohl (1980) Toxikologie, Pharmakokinetik und Interaktionen von Uromitexan. In: Burkert H, Nagel GR (eds) Neue Erfahrungen mit Oxazaphosphorinen unter besonderer Berücksichtigung des Uroprotektors Uromitexan. Karger, Basel, Beiträge zur Onkologie Bd 5, pp 12–20
Pohl J, Brock N, Niemeyer U, Scheffler G (1982) Pharmacological properties of ASTA Z 7557, a directly acting stabilized metabolite of cyclophosphamide. Proceedings 13th International Cancer Congress, Seattle USA, Abstract 2187, pp 384
Scheef W, Klein HO, Brock N, Burkert H, Günther N, Hoefer-Janker H, Mitrenga D, Schnitger J, Voigtmann R (1979) Controlled clinical studies with an antidote against the urotoxicity of oxazaphosphorines. Cancer Treat Rep 63:501–505
Shaw IC, Graham MI, McLean AEM (1983) 2-Chloroacetaldehyde: a metabolite of cyclophosphamide in the rat. Cancer Treat Rev 10 (Suppl A):17–24
Voelcker G, Jaschke A, Wrabetz H, Hohorst HJ (1984) Intracavitary high volume i.p. chemotherapy of S 180 ascites sarcoma in mice with 4-(S-ethanol)-sulfidocyclophosphamide in combination with protector thiolds. Arzneim Forsch 34:1291–1298
Wagner T, Heydrich D, Jork T, Voelcker G, Hohorst HJ (1981) Comparative study on human pharmacokinetics of activated ifosfamide and cyclophosphamide by a modified fluorometric test. J Cancer Res Clin Oncol 100:95–104
Wagner T, Mittendorff F, Walter E (1986) Intracavitary chemotherapy with activated cyclophosphamides and simultaneous systemic detoxification with protector thiols in sarcoma 180 ascites tumor. Cancer Res 46:2214–2219
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wagner, T., Zink, M. & Schwieder, G. Influence of mesna and cysteine on the systemic toxicity and therapeutic efficacy of activated cyclophosphamide. J Cancer Res Clin Oncol 113, 160–165 (1987). https://doi.org/10.1007/BF00391439
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00391439