Abstract
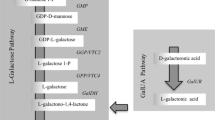
Detached leaves of Parthenocissus quinquefolia L., Vitaceae convert d-glucose to l-ascorbic acid with conservation of the carbon chain sequence and with retention of the hydroxymethyl group at carbon 6. l-Ascorbic acid is cleaved between carbons 4 and 5. The C4 fragment is converted to l-tartaric acid. The C2 fragment, possibly glycolaldehyde, recycles into products of hexose phosphate metabolism. During the metabolic period a relatively high portion of carbon-1 of l-ascorbic acid, as compared with carbon-4, was released as CO2. These studies demonstrate the usefulness of Virginia Creeper for yeararound research on ascorbic-acid metabolism and tartaric-acid biosynthesis in Vitaceae-type plants.
Similar content being viewed by others
Abbreviations
- AA:
-
l-Ascorbic Acid
References
Bloom, B. (1962) The simultaneous determination of 14C and 3H in the terminal groups of glucose. Anal. Biochem. 3, 85–87
Fricke, U. (1975) Tritosol: A new scintillation cocktail based on Triton X-100. Anal. Biochem. 63, 555–558
Kisaki, T., Yoshida, T., Imai, A. (1971) Glycine decarboxylase and serine formation in spinach leaf mitochondrial preparation with reference to photorespiration. Plant Cell Physiol. 12, 275–288
Kotera, U., Kodama, T., Minoda, Y., Yamada, K. (1972) Isolation and chemical structure of a new oxidation product of 5-ketogluconic acid, and a hypothetical pathway from glucose to tartaric acid through this new compound. Agric. Biol. Chem. 36, 1315–1325
Levi, C., Perchorowicz, J.T., Gibbs, M. (1978) Malate synthesis by dark carbon dioxide fixation in leaves. Plant Physiol. 61, 477–480
Loewus, F.A. (1963) Tracer studies on ascorbic acid formation in plants. Phytochemistry 2, 109–128
Loewus, F.A. (1980) l-Ascorbic acid: metabolism, biosynthesis, function. In: Biochemistry of plants, Stumpf, P.K., Conn, E.E., eds., vol. 3, Carbohydrates: structure and function, pp. 77–99, Preiss, J., ed. New York: Academic Press
Loewus, F.A., Baig, M.M. (1970) Biosynthesis and degradation of isotopically labeled ascorbic acid. Meth. Enzymol. 18, 22–29
Loewus, F.A., Helsper, J.P.F.G. (1981) Metabolism of l-ascorbic acid in plants. In: Ascorbic acid: chemistry, metabolism, uses, Seib, P.A., Tolbert, B.M. eds. Adv. Chem. Ser., Am. Chem. Soc., Washington, D.C. (in press)
Loewus, F.A., Jang, R., Seegmiller, C.G. (1956) The conversion of C14-labeled sugars to l-ascorbic acid in ripening strawberries. J. Biol. Chem. 222, 649–664
Loewus, F.A., Finkle, B.J., Jang, R. (1958) l-Ascorbic acid: A possible intermediate in carbohydrate metabolism in plants. Biochim. Biophys. Acta 30, 629–635
Loewus, F.A., Wagner, G., Yang, J.C. (1975) Biosynthesis and metabolism of ascorbic acid in plants. Ann. N.Y. Acad. Sci. 258, 7–23
Nakai, T., Ota, T., Wanaka, N., Bepper, D. (1974) Thin layer chromatographic detection of glycolaldehyde using a fluorescent reaction with o-aminodiphenyl. II. An improved procedure. J. Chromatogr. 88, 356–360
Ribéreau-Gayon, G. (1968) A study of the mechanism of synthesis and conversion of malic acid, tartaric acid and citric acid in Vitis vinifera L. Phytochemistry 7, 1471–1482
Rosenfield, C-L., Fann, C., Loewus, F.A. (1978) Metabolic studies on intermediates in the myo-inositol oxidation pathway in Lilium longiflorum pollen. I. Conversion to hexoses. Plant Physiol. 61, 89–95
Ruffner, H., Rast, D. (1974) The biogenesis of tartaric acid in the grapevine. Z. Pflanzenphysiol. 73, 45–55
Saito, K., Loewus, F.A. (1979) The metabolism of l-[6-14C]ascorbic acid in detached grape leaves. Plant Cell Physiol. 20, 1481–1488
Sprinson, D.B., Chargaff, E. (1946) On oxidative decarboxylations with periodic acid. J. Biol. Chem. 164, 433–449
Stafford, H.A., Loewus, F.A. (1958) The fixation of C14O2 into tartaric and malic acids of excised grape leaves. Plant Physiol. 33, 194–199
Tolbert, N.E. (1971) Microbodies-peroxisomes and glyoxysomes. Annu. Rev. Plant Physiol. 22, 45–74
Wagner, G., Loewus, F.A. (1973) The biosynthesis of (+)-tartaric acid in Pelargonium crispum. Plant Physiol. 52, 651–654
Wagner, G., Loewus, F.A. (1974) l-Ascorbic acid metabolism in Vitaceae. Conversion to (+)-tartaric acid and hexoses. Plant Physiol. 54, 784–787
Williams, M., Loewus, F.A. (1978a) Biosynthesis of (+)-tartaric acid from l-[4-14C]ascorbic acid in grape and geranium. Plant Physiol. 61, 671–674
Williams, M., Loewus, F.A. (1978b) A general procedure for the preparation of labeled l-ascorbic acid from labeled d-glucose. Carbohydr. Res. 63, 149–155
Williams, M., Saito, K., Loewus, F.A. (1979) Ascorbic acid metabolism in geranium and grape. Phytochemistry 18, 953–956
Zelitch, I. (1972) Comparison of the effectiveness of glycolic acid and glycine as substrates for photorespiration. Plant Physiol. 50, 109–113
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Helsper, J.P.F.G., Saito, K. & Loewus, F.A. Biosynthesis and metabolism of l-ascorbic acid in virginia creeper (Parthenocissus quinquefolia L.). Planta 152, 171–176 (1981). https://doi.org/10.1007/BF00391190
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00391190