Abstract
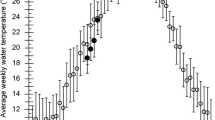
Laboratory culture of 40 Octopus bimaculoides from April 1982 to August 1983 through the full life cycle at 18°C vs 23°C provided information on the growth, reproductive biology and life span of this California littoral octopus. At 18°C, the cephalopods grew from a hatchling size of 0.07 g to a mean of 619 g in 404 d; the largest individual was 872 g. Octopuses cultured at 23°C reached their highest mean weight of 597 g in 370 d; the largest individual grown at this temperature was 848 g after 404 d. Growth data revealed a two-phase growth pattern: a 5 mo exponential phase followed by a slower logarithmic (power function) phase until spawning. At 5 mo octopuses grown at 23°C were over three times larger than their 18°C siblings. However, beyond 6.5 mo, growth rates were no higher at 23°C than at 18°C. At 13.5 mo, the mean weight of the 18°C group surpassed that of the 23°C group. The slope of the length/weight (L/W) relationship was significantly different for the two temperature regimes, with the 23°C octopuses weighing 18% less than their 18°C siblings at a mantle length of 100 mm. Females weighed more than males at any given mantle length. Males grew slightly larger and matured before females. The L/W relationship indicated isometric body growth throughout the life cycle. Higher temperature accelerated all aspects of reproductive biology and shortened life span by as much as 20% (from approximately 16 to 13 mo). O. bimaculoides has one of the longest life cycles among species with large eggs and benthic hatchlings. Extrapolations to field growth are made, and the possible effects of temperature anomalies such as El Niño are discussed.
Similar content being viewed by others
Literature cited
Ambrose, R. F. (1984). Food preferences, prey availability, and the diet of Octopus bimaculatus Verrill. J. exp. mar. Biol. Ecol. 77: 29–44
Ambrose, R. F. (1986). Effects of octopus predation on motile invertebrates in a rocky subtidal community. Mar. Ecol. Prog. Ser. 30: 261–273
Ambrose, R. F. (1988). Population dynamics of Octopus bimaculatus: influence of life history patterns synchronous reproduction and recruitment. Malacologia 29 (1): 23–29
Boletzky, S. von (1974). The “larvae” of Cephalopoda: a review. Thalassia jugosl. 10: 45–76
Boyle, P. R. (ed.) (1983). Cephalopod life cycles, Vol. I. Species accounts. Academic Press, London
Boyle, P. R. (ed.) (1987) Cephalopod life cycles, Vol. II, Comparative reviews. Academic Press, London
Brody, S. (1945). Bioenergetics and growth with special reference to the efficiency complex in domestic animals. Hafner Press, New York
Brown, M. E. (1957). Experimental studies on growth. In: Brown, M. E. (ed.) The physiology of fishes, Vol. 1, Metabolism. Academic Press, New York, p. 361–400
DeRusha, R. H., Forsythe, J. W., Hanlon, R. T. (1988). Laboratory growth, reproduction and life span of the Pacific pygmy octopus, Octopus digueti. Pacif. Sci. 41: 51–59
Dorsey, E. M. (1976). Natural history and social behavior of Octopus rubescens Berry. Master's thesis, University of Washington, Friday Harbor
Forsythe, J. W. (1984). Octopus joubini (Mollusca: Cephalopoda): a detailed study of growth through the full life cycle in a closed seawater system. J. Zool., Lond. 202: 393–417
Forsythe, J. W., Hanlon, R. T. (1988). Behavior, body patterning and reproductive biology of Octopus bimaculoides from California. Malacologia 29 (1): 40–56
Forsythe, J. W., Van Heukelem, W. F. (1987). Cephalopod growth. In: Boyle, P. R. (ed.) Cephalopod life cycles, Volume II, Comparative reviews. Academic Press, London, p. 135–155
Gould, S. J. (1966). Allometry and size in ontogeny and phylogeny. Biol. Rev. 41: 587–640
Guerra, A. (1979). Fitting a von Bertalanffy expression to Octopus vulgaris growth. Investigación pesq. 43: 319–329
Hanlon, R. T. (1983). Octopus briareus. In: Boyle, P. R. (ed.) Cephalopod life cycles, Vol. I. Species accounts. Academic Press, London, p. 251–266
Hanlon, R. T., Forsythe, J. W. (1985). Advances in the laboratory culture of octopuses for biomedical research. Lab. Anim. Sci. 35: 33–40
Hartwick, B. (1983). Octopus dofleini. In: Boyle, P. R. (ed.) Cephalopod life cycles, Vol. 1, Species accounts. Academic Press, London, p. 277–291
Hendrix, J. P., Jr., Hulet, W. H., Greenberg, M. J. (1981). Salinity tolerance and the responses to hypoosmotic stress of the bay squid Lolliguncula brevis, a euryhaline cephalopod mollusc. Comp. Biochem. Physiol. 69A: 641–648
Hochberg, F. G., Jr., Fields, W. G. (1980). The squids and octopuses. In: Morris, R. H., Abbott, D. P., Haderlie, E. C. (eds.) Intertidal invertebrates of California, Chapter 17: Cephalopoda. Stanford, Stanford University Press, p. 429–444 and P133-P136
Mangold, K. (1983). Octopus vulgaris. In: Boyle, P. R. (ed.) Cephalopod life cycles, Vol. 1, Species accounts. Academic Press, London, p. 335–364
McGowan, J. A. (1984). The California El Niño, 1983. Oceanus 27 (2): 48–51
Packard, A. (1972). Cephalopods and fish: the limits of convergence. Biol. Rev. 47: 241–307
Pascual, E. (1978). Crecimiento y alimentación de tres generaciónes de Sepia officinalis en cultivo. (Growth and feeding of three generations of lab-reared Sepia officinalis). Investigación pesq. 42: 421–442
Pickford, G. E., McConnaughey, B. H. (1949). The Octopus bimaculatus problem: a study in sibling species. Bull. Bingham oceanogr. Coll. 12(4): 1–66
Richard, A. (1966). Action de la temperature sur l'evolution genitale de Sepia officinalis L. C. r. hebd. Séanc. Acad. Sci., Paris 263: 1998–2001
Ricker, W. E. (1979). Growth rates and models. Fish Physiol. 8: 677–743
Van Heukelem, W. F. (1976). Growth, bioenergetics and life-span of Octopus cyanea and Octopus maya. Ph.D. dissertation, University of Hawaii, Honolulu
Van Heukelem, W. F. (1979). Environmental control of reproduction and life span in Octopus: an hypothesis. In: Stancyk, S. E. (ed.) Reproductive ecology of marine invertebrates. University of South Carolina Press, Columbia, p. 123–133
Wells, M. J., Wells, J. (1970). Observations on the feeding, growth rate and habits of newly settled Octopus cyanea. J. Zool., Lond. 161: 65–74
Young, R. E., Harman, R. F. (1988). “Larva”, “paralarva” and “subadult” in cephalopod terminology. Malacologia 29 (1): 201–207
Zar, J. H. (1974). Biostatistical analysis. Prentice-Hall Inc., Englewood Cliffs
Author information
Authors and Affiliations
Additional information
Communicated by R. S. Carney, Baton Rouge
Rights and permissions
About this article
Cite this article
Forsythe, J.W., Hanlon, R.T. Effect of temperature on laboratory growth, reproduction and life span of Octopus bimaculoides . Marine Biology 98, 369–379 (1988). https://doi.org/10.1007/BF00391113
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00391113