Summary
Reproductive capacities of tropical and temperate populations of D. melanogaster were compared using three complementary techniques: (1) measure of egg production by females grown in the laboratory under uncrowded conditions and provided as adults with abundant food; (2) study of egg production of flies of unknown ages, collected in nature and then kept in similar conditions; and (3) analysis of ovarian activity of wild females dissected just after their capture.
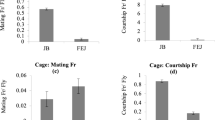
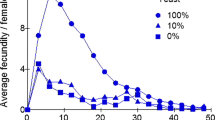
Tropical populations showed a lower fecundity in the laboratory and this was also observed in laboratory reared adults. On the average, flies also appeared to be older in the tropics than in temperate countries. These data, together with ecological observations showing that tropical populations live in a more predictable and stable environment, suggest that temperature populations are r-selected, while tropical ones are K-selected. The study of ovarian activity of wild females failed however to confirm this expectation. Tropical flies, which have a lower genetic fecundity, generally appeared to produce more propagules than did temperate flies. Such a contradiction shows how the ideas of r- and K-selection are difficult to apply to natural populations of Drosophila. Population density and interindividual competition are probably not the main selective forces in nature. Attention must also be paid to the necessity of exploring the environment to find resources, to the role of predation and parasitism, and to the occurrence in temperate countries of seasonal fluctuations with different selective pressures on successive generations.
Similar content being viewed by others
References
Allemand R, David JR (1976) The circadian rhythm of oviposition in Drosophila melanogaster: a genetic latitudinal cline in wild populations. Experientia 32:1403
Atkinson WD (1979) A field investigation of larval competition in domestic Drosophila. J Anim Ecol 48:91–102
Barbosa (1977) r and K strategies in some larval and pupal parasitoids of Gypsy moth. Oecologia 29:311–327
Barclay HJ, Greogory PT (1981) An experimental test of models predicting life-history characteristics. Amer Nat 117:944–961
Boulétreau J (1974) Importance relative des stimulations de l'accouplement: parade, copulation et insémination sur la production ovarienne de Drosophila melanogaster. Bull Biol 108:61–70
Boulétrau J (1978) Ovarian activity and reproductive potential in a natural population of Drosophila melanogaster. Oecologia (Berl) 35:319–342
Boulétreau-Merle J (1975) Influence de l'accouplement sur la physiologie reproductrice des femelles de Drosophila melanogaster. Thèse Doctorat d'Etat, Lyon, 320 p
Carton Y, Kitano H (1981) Evolutionary relationships to parasitism by seven species of the Drosophila melanogaster subgroup. Biol J Linnean Soc (in press)
Cohet Y (1975) Epigenetic influences on the life span of the Drosophila: existence of an optimal growth temperature for adult longevity. Exptl Geront 10:181–184
Cohet Y, David JR (1978) Control of the adult reproductive potential by preimaginal thermal conditions: a study in Drosophila melanogaster. Oecologia (Berl) 36:295–306
Cohet Y, Vouidibio J, David JR (1980) Thermal tolerance and geographic distribution: a comparison of cosmopolitan and tropical endemic Drosophila species. J Therm Biol 5:69–74
David J (1970) Le nombre d'ovarioles chez Drosophila melanogaster: relation avec la fécondité et valeur adaptative. Arch Zool Exp Gén 111:357–370
David JR (1979) Utilization of morphological traits for the analysis of genetic variability in wild populations. Aquilo, Ser Zool 20:49–61
David J, Biemont C, Fouillet P (1974) Sur la forme des courbes de ponte de Drosophila melanogaster et leur ajustement à des modèles mathématiques. Arch Zool Exp Gén 115:263–277
David JR, Bocquet C (1975) Similarities and differences in latitudinal adaptation of two Drosophila sibling species. Nature 257:588–590
David J, Bocquet C, De Scheemaeker-Louis M (1977) Genetic latitudinal adaptation of Drosophila melanogaster: new discriminative biometrical traits between European and equatorial Africa populations. Genet Res Camb 30:247–255
David JR, Cohet Y, Fouillet P, Arens MF (1980) Phenotypic variability of wild collected Drosophila: an approach toward understanding selective pressures in natural populations. Egyp J Genet Cytol 9:51–66
David JR, Tsacas L (1981) Cosmopolitan, subcosmopolitan and wide-spread species: different strategies within the drosophilid family (Diptera) CR Soc Biogéog 57:11–26
Dobzhansky T (1950) Evolution in the tropics. Am Sci 38:209–221
Foster MS (1974) A model to explain molt-breeding overlap and clutch size in some tropical birds. Evolution 28:182–190
Giesel JT (1976) Reproductive strategies as adaptations to life in temporally heterogeneous environments. Ann Rev Ecol System 7:57–79
Ives PT (1970) Further genetic studies of the South Amherst population of Drosophila melanogaster. Evolution 24:507–518
Kambysellis ML, Heed WB (1971) Studies of oogenesis in natural populations of Drosophilidae I: Relation of ovarian development and ecological habitats of the Hawaiian species. Am Nat 105: 31–49
King RC, Rubinson AC, Smith RF (1956) Oogenesis in adult Drosophila melanogaster. Growth 20:121–157
Lachaise D (1974) Les drosophilidae des savanes préforestières de la région tropicale de Lamto (Côte d'Ivoire) I — Isolement écologique des espèces affines et sympatriques; rythmes d'activité saisonnière et circadienne; rôle des feux de brousse. Ann Univ Abidjan, Série E 7:7–152
L'Heritier P, Teissier G (1933) Etude d'une population de Drosophiles en équilibre. CR Acad Sci (Paris) 197:1765–1767
Lints FA, Lints CV (1971) Influence of preimaginal environment on fecundity and ageing in Drosophila melanogaster hybrids. II: Preimaginal temperature. Exp Geront 6:417–426
Maiorana VC (1976) Predation, submergent behaviour and tropical diversity. Evol Theory 1:157–177
MacArthur RC, Wilson EO (1967) The theory of Island Biogeography. Princeton Univ Press, Princeton, 203 p
Parsons PA (1980a) Adaptative strategies in natural populations of Drosophila. Theor Appl Genet 57:257–266
Parsons PA (1980b) Isofemale strains and evolutionary strategies in natural populations. Evol Biol 13:175–217
Petit C (1969) L'explosion démographique annuelle et la structure génétique des populations de Drosophila melanogaster. Bull Soc Zool Fr 94:641–648
Pianke E (1970) On “r” and “K” selection. Am Nat 104:592–597
Rouault J (1979) Rôle des parasites entomophages dans la compétition entre espèces jumelles de drosophiles: approche expérimentale. CR Acad Sc 289:643–646
Shorrocks B, Charlesworth P (1980) The distribution and abundance of the British fungal-breeding Drosophila. Ecolog Entom 5:61–78
Stearns SG (1976) Life-history tactics: a review of the ideas. Quart Rev Biol 51:3–47
Taylor CE, Condra C (1980) r- and K-selection in Drosophila pseudoobscura. Evolution 34:1183–1193
Tsacas L, Lachaise D (1974) Quatre nouvelles espèces de la Côte d'Ivoire du genre Drosophila, groupe melanogaster et discussion de l'origine du sous-groupe melanogaster (Diptera; Drosophilidae) Ann Univ Abidjan, Sci E (Ecologie) 7:193–211
Van Herrewege J (1970) Intervention de stimuli olfactifs dans l'oviposition de la Drosophile. CR Acad Sci (Paris) 271:108–110
Vouidibio J (1977) Biologie évolutive et écophysiologie comparée de deux espèces de Drosophila africains: D. iri et D. fraburu. Thèse Doctorat spécialité, Lyon, 144
Wilbur HM, Tinkle DW, Collins JP (1974) Environmental certainty, trophic level, and resource availability in life history evolution. Am Nat 108:805–817
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boulétreau-Merle, J., Allemand, R., Cohet, Y. et al. Reproductive strategy in Drosophila melanogaster: Significance of a genetic divergence between temperate and tropical populations. Oecologia 53, 323–329 (1982). https://doi.org/10.1007/BF00389008
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00389008