Abstract
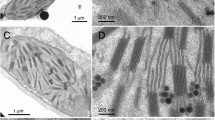
Segments of 7-d low light-grown barley laminae cut at 0.5 cm intervals up from the intercalary meristem were examined ultrastructurally and biochemically. The different regions upwards showed the succession of plastid development in light-grown tissues of eoplasts, amyloplasts, amoeboid, immature and mature plastids as described by Whatley (1977). Semi-crystalline bodies were detected in all of them. The eoplast-amyloplast regions are characterised by a greater proportion of mitochondria and high levels of ATP and 3-phosphoglyceric acid, together with low levels of inorganic phosphate conducive to the activation of ADP glucose pyrophosphorylase. The amoeboid and immature plastid regions have higher levels of inhibitory phosphate and starch breakdown may be responsible for the release of metabolites and energy for development. Segments containing amoeboid and immature plastids also have reduced levels of ATP (and 3-phosphoglyceric acid) as photosynthetic components are synthesised. Using ultrastructural assessments of areas of thylakoids, first β-carotene and violaxanthin, followed by chlorophyll a and lutein and, lastly, chlorophyll b are concentrated in the developing lamellar systems of the immature and mature chloroplasts. The formation of additional membraneous material which spreads these pigment systems over a greater thylakoid area within the plastids is the final stage of plastid morphogenesis in low light-grown seedlings.
Similar content being viewed by others
Abbreviations
- Chl:
-
chlorophyll
- 3-PGA:
-
3 phosphoglyceric acid
References
Abbott, I.R., Matheson, N.K. (1972) Starch depletion in germinating wheat, wrinkled-seeded peas and senescing tobacco leaves. Phytochemistry 11, 1261–1272
Baker, N.R., Leech, R.M. (1977) Development of photosystem I and photosystem II activities in light-grown maize (Zea mays). Plant Physiol 60, 640–644
Boffey, S.A., Selldén, G., Leech, R.M. (1980) Influence of cell age on chlorophyll formation in light-grown and etiolated wheat seedlings. Plant Physiol 65, 680–684
Chapman, D.J., Leech, R.M. (1979) Changes in pool sizes of free amino acids and amides in leaves and plastids of Zea mays during leaf development. Plant Physiol. 63, 567–572
Esau, K. (1953) Plant Anatomy. Wiley & Sons. New York
Gaudillère, J.P. (1974) Amelloration du dosage spectrophotometrique des chlorophyll a et b et des caroténoides totaux dans des extracts foliares. Physiol. Veg. 12, 585–599
Ghosh, H.P., Preiss, J. (1966) Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of spinach leaf chloroplasts. J. Biol. Chem. 241, 4491–4504
Hampp, R., Wellburn, A.R. (1980) Translocation and phosphorylation of adenine nucleotides by mitochondria and plastids during greening. Z. Pflanzenphysiol. 98, 289–303
Hampp, R., Ziegler, H. (1980) On the use of Avena protoplasts to study chloroplast development. Planta 147, 485–494
Hawke, J.C., Rumsby, M.G., Leech, R.M. (1974) Lipid biosynthesis in green leaves of developing maize. Plant Physiol. 53, 555–561
Kasemir, H. (1979) Control of chloroplast formation by light. Cell Biol. Int. Rep. 3, 197–214
Kesselmeier, J., Budzikiewicz, H. (1979) Identification of saponins as structural building units in isolated prolamellar bodies from etioplasts of Avena sativa L. Z. Pflanzenphysiol. 91, 333–344
Leese, B.M., Leech, R.M., Thompson, W.W. (1971) Isolation of plastids from different regions of developing maize leaves. In: Proc. of IInd Int. Congress on Photosynthesis, vol. 3, pp. 1485–1493, Forti, G., Avron, M., Melandin, A. eds Junk, The Hague
Lichtenthaler, H.K., Burkard, G., Kuhn, G., Prenzel, U. (1981) Licht-induced accumulation and stability of chlorophylls and chlorophyll-proteins during chloroplast development in radish seedlings. Z. Naturforsch. 36, 421–420
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. (1951) Protein measurement with Folin Phenol reagent. J. Biol. Chem. 193, 265–275
Newcomb, E.H., Mersey, B.G., Cline, K.C. (1981) Plastid tubules associated with the inner membrane of the chloroplast envelope. In: Proc. of Vth Int. Congress on Photosynthesis, Akoyunoglou, G., ed. (in press)
Ozbun, I.L., Hawker, J.S., Preiss, J. (1972) Soluble adenosine diphosphate glucose-α-1,4-glucan-α-4-glycosyl-transferase from spinach leaves. Biochem. J. 126, 953–963
Platt-Aloia, K.A., Thomson, W.W. (1977) Chloroplast development in young seasame plants. New Phytol. 78, 599–605
Rascio, N., Orsenigo, M., Arboit, D. (1976) Prolamellar body transformation with, increasing cell age in the maize leaf. Protoplasma 90, 253–263
Robertson, D., Laetsch, W.M., (1974) Structure and function of developing barley plastids. Plant Physiol. 54, 148–159
Taussky, H.H., Shorr, E. (1953) A microcolourimetric method for the determination of inorganic phosphorous. J. Biol. Chem. 202, 675–685
Wellburn, A.R., Hampp, R. (1979) Appearance of photochemical function in prothylakoids during plastid development. Biochim. Biophys. Acta 547, 380–397
Wellburn, A.R., Higginson, C., Robinson, D., Walmsley, C. (1981) Biochemical explanations of more than additive inhibitory effects of low atmospheric levels of sulphur dioxide plus nitrogen dioxide upon plants. New Phytol. 88, 223–237
Wellburn, A.R., Quail, P.H., Gunning, B.E.S. (1977) Examination of ribosome-like particles in isolated prolamellar bodies. Planta 134, 45–52
Wellburn, F.A.M., Wellburn, A.R. (1979) Conjoined mitochondria and plastids the barley mutant ‘Albostrians’. Planta 147, 178–179
Wellburn, F.A.M., Wellburn, A.R., Senger, H. (1980) Changes in ultrastructure and photosynthetic capacity within Scenedesmus obliquus mutants C-2A′, C-6D and C-6E on transfer from dark grown to illuminated conditions. Protoplasma 103, 35–54
Whatley, J.M. (1974) Chloroplast development in primary leaves of Phaseolus vulgaris. New Phytol. 78, 1097–1110
Whatley, J.M. (1977) Variations in the basic pathway of chloroplast development. New Phytol. 78, 407–420
Wrischer, M. (1966) Neubildung von Prolamellarkörpern in Chloroplasten. Z. Pflanzphysiol. 55, 296–299
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wellburn, A.R., Robinson, D.C. & Wellburn, F.A.M. Chloroplast development in low light-grown barley seedlings. Planta 154, 259–265 (1982). https://doi.org/10.1007/BF00387872
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00387872