Summary
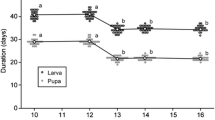
The response by male and female plants to herbivory was studied by experimental defoliation of the dioecious perennial herb Silene dioica in a green-house. Male and female plants were defoliated prior to and during the early flowering phase at two intensities (50% and 100% of leaf-area removed) in two consecutive years. Defoliation resulted in a decrease in the number of flowers initiated in both sexes, while a larger delay of peak flowering and a higher mortality was observed in males compared to females. In female plants, severe defoliation resulted in a reduction in seed number per capsule and in seed size compared to control. Females showed a negative correlation between the production of flowers in the first and second season in all treatments, while flowering in males the first season was not correlated with flowering in the second season. Females also showed a lower frequency of flowering than males during the two seasons studied. However, during the flowering period, males allocated significantly more biomass to flowers than did females. This outcome supports the idea that females may have a higher total reproductive expenditure than males, but males have a higher reproductive effort during flowering. Male rosette leaves were significantly preferred by the generalist herbivore Arianta arbustorum in experiments. This preference was most pronounced in trials with leaves from fertilized plants compared to nonfertilized plants. A greater storage of resources in aboveground leaves during winter by males compared to females may explain the higher preference for male leaves and the higher male mortality following early defoliation. Furthermore, males are smaller than females and may have a lower ability than females to replace lost resources needed for reproduction when defoliated early in the season.
Similar content being viewed by others
References
Ågren J (1987) Intersexual differences in phenology and damage by herbivores and pathogens in dioecious Rubus chamaemorus L. Oecologia (Berlin) 72:161–169
Ågren J, Elmqvist T, Tunlid A (1986) Pollination by deceit, floral sex ratios and seed set in dioecious Rubus chamaemorus L. Oecologia (Berlin) 70:332–338
Baker HG (1947) Biological flora of the British Isles. Melandrium (Roehling em.) Fries. J Ecol 35:271–292
Bawa KS (1980a) Evolution of dioecy in flowering plants. Anno Rev Ecol Syst 11:15–39
Bawa KS (1980b) Mimicry of male by female flowers and intrasexual competition for pollinators in Jacaratia dolichaula (D. Smith) Woodson (Caricaceae). Evolution 34:467–474
Bawa KS, Opler PA (1975) Dioecism in tropical trees. Evolution 29:167–179
Bazzaz FA, Chiariello NR, Coley PD, Pitelka LF (1987) Allocating resources to reproduction and defense. Bio Science 37:58–67
Bierzychudek P (1987) Pollinators increase the cost of sex by avoiding female flowers. Ecology 68:444–447
Bourdeau PF (1958) Photosynthetic and respiratory rates in leaves of male and female quaking aspen. For Sci 4:331–334
Bridel M, Beguin C (1926) Recherches biochimiques sur la composition du Salix triandra L. C R Acad Sci 183:231–233
Conn JS (1981) Phenological differentiation between the sexes of Rumex hastatulus: niche partitioning or different optimal reproductive strategies? Bull Torrey Bot Club 108:374–378
Correns C (1928) Bestimmung, Vererbung und Verteilung des Geschlechtes bei den höheren Pflanzen. In: Baur E, Hartmann M (eds) Handb Vererbungsw 2:1–128
Cox PA (1981) Niche partitioning between sexes of dioecious plants. Am Nat 117:295–307
Cox PA (1988) Monomorphic and dimorphic sexual strategies: a modular approach. In: Lovett Doust J, Lovett Doust L (eds) Plant reproductive ecology: strategies and patterns. Oxford University Press, Oxford, pp 80–97
Crawley MJ (1983) Herbivory: the dynamics of animal-plant interactions. Blackwell Scientific Publications, Oxford
Danell K, Elmqvist T, Ericson L, Salomonson A (1985) Sexuality in willows and preferences by bark-eating voles: defence or not? Oikos 44:82–90
Endler JA (1978) A predator's view of animal color patterns. Evol Biol 11:319–364
Freeman DG, Klikhoff LG, Harper KT (1976) Differential resource utilization by the sexes of dioecious plants. Science 193:597–599
Grant MC, Mitton JB (1979) Elevational gradients in adult sex ratios and sexual differentiation in vegetative growth rates of Populus tremuloides Michx. Evolution 33:914–918
Gross KL, Soulé JD (1981) Differences in biomass allocation to reproductive and vegetative structures of male and female plants of a dioecious perennial herb Silene alba (Miller) Krause. Am J Bot 68:801–807
Hadley EB, Bliss LC (1964) Energy relationships of alpine plants on Mt. Washington, New Hampshire. Ecol Monogr 34:331–357
Herrera CM (1984) The annual cycle of Osyris quadripartita a hemiparasitic dioecious shrub of Mediterranean scrublands. J Ecol 72:1065–1078
Janzen DH (1976) Effect of defoliation on fruit-bearing branches of the Kentucky coffee tree Gymnocladius dioicus (Leguminosae). Am Mid Nat 95:474–478
Kay QON, Lack AJ, Bamber FC, Davies CR (1984) Differences between sexes in floral morphology, nectar production and insect visits in a dioecious species Silene dioica. New Phytol 98:515–529
Lawrence CW (1963) Genetic studies on wild populations of Melandrium. II Flowering time and plant weight. Heredity 18:149–163
Lloyd DG (1980) Sexual strategies in plants. I. An hypothesis of serial adjustment of maternal investment during one reproductive session. New Phytol 86:69–79
Lloyd DG, Webb CJ (1977) Secondary sex characters in seed plants. Bot Rev 43:177–216
Loehwing WF (1933) Physico-chemical aspects of sex in plants. Proc Soc Exp Biol Med 30:1215–1220
Lovett Doust J, Lovett Doust L (1985) Sex ratios, clonal growth and herbivory in Rumex acetosella. In: White J (ed) Studies on plant demography. Academic Press, London
Lovett Doust L, Lovett Doust J (1987) Leaf demography and clonal growth in female and male Rumex acetosella. Ecology 68:2056–2058
Lovett Doust J, O'Brian GA, Lovett Doust L (1987) Effect of density on secondary sex characteristics and sex ratio in Silena alba (Caryophyllaceae). Am J Bot 74:40–46
Maun MA, Cavers PB (1971) Seed production in Rumex crispus. The effects of removal of cauline leaves at anthesis. Can J Bot 49:1123–1130
Meagher TR, Antonovics JJ (1982) Life history variation in dioecious plant populations: a case study of Chamaelirium luteum. In: Dingle H, Hegmann JP (eds) Evolution and genetics of life histories. Springer Berlin Heidelberg New York, pp 139–154
Neverova LA (1971) Sootnošenie raznopolych osobej i soderžanie u nich tanidov v nekotorych vidov ivy v okrestnostjach Uralska. Rastit Resur 7:77–80 (in Russian)
Nigtevecht G van (1966) Genetic studies in dioecious Melandrium I. Sex-linked and sex-influenced inheritance in Melandrium album and Melandrium dioicum. Genetica 37:281–306
Reader RJ (1978) Contribution of overwintering leaves to the growth of three broad-leaved, evergreen shrubs belonging to the Ericaceae family. Can J Bot 56:1248–1261
Stanfield JF (1937) Certain physico-chemical aspects of sexual differentiation in Lychnis dioica. Am J Bot 24:710–719
Stanfield JF (1944) Chemical composition of roots and tops of dioecious Lychnis in vegetative and flowering phases of growth. Plant Physiol 19:377–383
Thieme H (1965) Die Phenolglykoside der Salicaceen. Pharmazie 20:570–574
Thornhill R (1976) Sexual selection and nuptial feeding behaviour in Bittacus apicalis (Insecta: Mecoptera). Am Nat 110:529–548
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Elmqvist, T., Gardfjell, H. Differences in response to defoliation between males and females of Silene dioica . Oecologia 77, 225–230 (1988). https://doi.org/10.1007/BF00379190
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00379190