Abstract
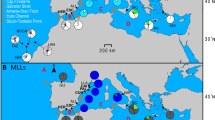
Allelic and genotypic frequencies were determined for samples from 35 widely distributed Australasian colonies of Actinia tenebrosa and 2 South African colonies of A. equina. These data provided no evidence of gene flow between Australisian and South African Actinia colonies and indicated that there may be some restriction of gene flow between widely separated Australasian colonies.
Both species are viviparous, and brooded A. tenebrosa are known to be produced asexually. The present data indicate that, within both species, almost all genotypic diversity is generated by sexual reproduction with recombination. Sexually produced juveniles appear to be widely dispersed and panmixis may occur over thousands of kilometres. However, successful sexual recruitment must be episodic or rare. Colonies on stable shores displayed relatively low levels of genotypic diversity, as compared with expectations for sexually reproducing populations, indicating strong local effects of asexual recruitment. Clonal genotypes may be spread over hundreds of metres of shore, but are typically restricted to discrete colonies. Asexual recruitment is highly localised and asexual dispersal appears to be limited by lengths of shore (≤500 m) which are unsuitable for colonization. Colonies on unstable shores are significantly more diverse genotypically and show little evidence of clonal proliferation.
Similar content being viewed by others
References
Ayre DJ (1983a) The effects of asexual reproduction and intergenotypic aggression on the genotypic structure of populations of the sea anemone Actinia tenebrosa. Oecologia (Berlin) 57:158–165
Ayre DJ (1983b) The distribution of the sea anemone Actinia tenebrosa Farquhar in south-western Australia. West Aust Nat 15:136–140
Bell G (1982) The masterpiece of nature: the evolution and genetics of sexuality. London: Croon Helm
Black R, Johnson MS (1979) Asexual viviparity and population genetics of Actinia tenebrosa. Mar Biol 53:27–31
Bonner J (1958) The relation of spore formation to recombination. Amer Nat 92:193–200
Bucklin AC (1980) The reproduction and population biology of Metridium (Coelenterata, Actinaria) Ph.D. Thesis University of California, Berkeley
Carlgren O (1924) Actinaria from New Zealand and its subantartic islands. Papers from Dr. T. Mortensen's Pacific Expedition 1914–1916, 21. Vidensk Meddr dansk naturh Foren 77:179–161
Carlgren O (1939) South African Actinaria and Zoantharia. K Svenska Vetens Akad Handl 17:1–148
Cresswell GR, Golding TJ (1980) Observations of a south-flowing current in the south-eastern Indian Ocean. Deep-Sea Res 27A, 449–466
Crow JR, Kimura M (1965) Evolution in sexual and asexual populations. Am Nat 99:439–450
Ebert TA (1982) Recruitment in Echinoderms. In Echinodern studies Lawerence J, Jangoux M, Balkena AA (eds), Rotterdam, The Netherlands
Fadlallah YH (1982) Reproductive ecology of the coral Astrangia lajollaensis: Sexual and asexual patterns in a kelp forest habitat. Oecologia (Berlin) 55:379–388
Frank PW (1975) Latitudinal variation in the life history features of the Black Turban Snail tegula funebralis (Prosobranchia: Trochidae) Mar Biol 31:181–192
Hamon BV, Godfrey JS, Greig MA (1975) The relation between mean sea level, current and wind stress on the east coast of Australia. Aust J Mar Freshwat Res 26:389–403
Harris H, Hopkinson DA (1976) Handbook of Enzyme Electrophoresis in Human Genetics. North-Holland Publishing Co Inc Amsterdam
Hoffmann RJ (1976) Genetics and asexual reproduction of the sea anemone Metridium senile. Biol Bull 151:478–488
Loosanoff VL (1964) Variations in time and intensity of settling of the starfish, Asterias forbesi, in Long Island Sound during a twenty-five year period. Biol Bull 126:423–439
Markina NP (1976) Biogeographic regionalisation of Australian waters of the Indian Ocean. Oceanology 15:602–604
Maynard Smith J (1978) The evolution of sex. Cambridge University Press, Cambridge
Maxwell JGH, Creswell GR (1981) Dispersal oftropical marine fauna to the Great Australian Bight by the Leeuwin Current. Aust J Mar Freswhat Res 32:493–500
Nei M (1978) Estimation of Average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Ottaway JR (1973) Some effects of temperature desiccation and light on the intertidal anemone Actinia tenebrosa Farquhar (Cnidaria: Anthozoa) Aust J Mar Freshwat Res 24:103–126
Ottaway JR (1979a) Population ecology of the intertidal anemone Actinia tenebrosa II. Geographic distribution, synomony, reproductive cycle and fecundity. Aust J Zool 27:273–290
Ottaway JR (1979b) Population ecology of the intertidal anemone Actinia tenebrosa III. Dynamics and environmental factors. Aust J Mar Freshwat Res 30:41–62
Ottaway JR (1980) Population ecology of the intertidal anemone Actinia tenebrosa IV Growth rates and longevities. Aust J Mar Freshwat Res 31:385–395
Rochford DJ (1969) Seasonal variations in the Indian Ocean along 110° E I. Hydrological structure of the upper 500 m. Aust J mar Freshwat Res 20:1–50
Selander RK, Smith MH, Yang SY, Johnson WE, Gentry JB (1971) Biochemical polymorphism and systematics in the genus Peromyscus. I. Variation in the Old-Field Mouse (Peromyscus polionotus). Studies in Genetics VI. Univ Tex Publs 7103:49–90
Shick JM, Hoffmann RJ, Lamb AN (1979) Asexual reproduction population structure and genotype-environment interactions in sea anemones. Am Zool 19:699–713
Sokal RR, Sneath PHA (1963) Principles of numerical taxonomy. Freeman WH, London
Stoddart JA (1983) A genotypic diversity measure. J Hered 74:489
Underwood AJ (1979) The ecology intertidal gastropods. Adv Mar Biol 16:111–210
Williams GC (1975) Sex and Evolution. Princeton University Press, Princeton
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ayre, D.J. The effects of sexual and asexual reproduction on geographic variation in the sea anemone Actinia tenebrosa . Oecologia 62, 222–229 (1984). https://doi.org/10.1007/BF00379017
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00379017