Summary
From preliminary experiments it was known that radiolabelled benzene and some of its metabolites during its metabolic activation process produce different in vitro DNA-phenyladducts in mitoplasts [5, 11].
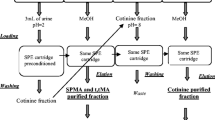
As we reported previously [9] at least one of these adducts, N-7-phenylguanine, is excreted in the urine of rats in measurable amounts, probably through an excision-repair mechanism after an inhalation experiment. Now we found, after i.p. application of benzene in the urine of rats, a compound separated by cation-exchange chromatography that behaves like a synthezised N-7-phenylguanine reference substance with respect to its retention index and the UV-absorption. This finding could be confirmed by HPLC-measurements with reversed-phase carrier materials. Silylation and gaschromatographic/mass spectrometric (GC/MS) separation of the fraction, which contains the phenylguanine, revealed that these fractions contain further phenyl adducts. Furthermore we studied the time-dependent excretion of the DNA-base adduct. Surprisingly the excretion dropped to zero on the fourth day and showed a new increase thereafter.
Similar content being viewed by others
References
Angerer J (1983) Prävention beruflich bedingter Gesundheitsschäden durch Benzol, Toluol, Xylole and Ethylbenzol. In: Schriftenreihe Arbeitsmedizin, Sozialmedizin, Präventivmedizin, Band 71, Gentner Verlag, Stuttgart, pp 89–90
Arfellini G, Grilli S, Colacci A, Mazzullo M, Prodi G (1985) In vivo and in vitro binding of benzene to nucleic acids and proteins of various rat and mouse tissues. Cancer Lett 28:159–168
Boyland E, Sims P (1962) Metabolism of polycyclic compounds, part 21. Biochem J 84:564–571
Gill DP, Ahmend AE (1981) Covalent binding of 14-Cbenzene to cellular organelles and bone marrow nucleic acids. Biochem Pharmacol 10:1127–1131
Kalf GF, Snyder R, Rushmore TH (1985) Inhibition of RNA synthesis by benzene metabolites and their covalent binding to DNA in rabbit bone marrow mitochondria in vitro. Am J Ind Med 7:485–492
Ludewig G, Glatt HR (1986) Mutations in bacteria and sister chromatid exchanges in cultured mammalian cells are induced by different metabolites of benzene. In: Deutsche Pharmakologische Gesellschaft, Spring Meeting March 11–14, 1986 Mainz
Lutz WK, Schlatter C (1977) Mechanism of the carcinogenic action of benzene: irreversible binding to rat liver DNA. Chem-Biol Interact 18:241–245
Müller G, Kölbel M, Heger M, Norpoth K (1987) Urinary S-phenylmercapturic acid and phenylguanine as indicators of benzene exposure. In: Ho MH, Dillon HK (eds) Biological monitoring of exposure to chemicals — organic compounds. John Wiley & Sons Inc, New York, pp 91–98
Müller G, Kolbel M, Norpoth K (1984) Phenylguanin ein Beanspruchungsparameter bei Benzolexposition? In: Konietzko H, Schuckmann F (eds) Verhandlungen der Deutschen Gesellschaft für Arbeitsmedizin, 24. Jahrestagung, Mainz. Gentner Verlag, Stuttgart, pp 319–323
Norpoth K (1984) Krebserzeugende Arbeitsstoffe: Benzol. In: Henschler D, Lehnert G (eds) Biologische Arbeitsstoff-Toleranz-Werte (BAT-Werte), Band 1. Verlag Chemie, Weinheim
Rushmore TH, Snyder R, Kalf GF (1984) Covalent binding of benzene and its metabolites to DNA in rabbit bone marrow mitochondria in vitro. Chem-Biol Interact 49:133–154
Sims P (1964) Metabolism of polycyclic compounds part 25. Biochem J 92:621–631
Snyder R, Lee EW, Kocsis JJ (1978) Binding of labeled benzene metabolites to mouse liver and bone marrow. Res Commun Chem Pathol Pharmacol 20:191–194
Tunek A, Platt KL, Bentley P, Oesch F (1978) Microsomal metabolism of benzene to species irreversible binding to microsomal proteins and effects of modification of this metabolism. Mol Pharmacol 14:920–929
Tunek A, Platt KL, Przybylski M, Oesch F (1980) Multistep metabolic activation of benzene. Effect of superoxide dismutase on covalent binding to microsomal macromolecules and identification of glutathione conjugates using high pressure liquid chromatography and field desorption mass spectrometry. Chem-Biol Interact 33:1–17
Verkoyen C, Golovinsky E, Müller G, Kölbel M, Norpoth K (1987) Arylsubstituierte Purine, I, Synthese von 7-Phenylguanin und 2-Substituierten 7-Arylhypoxanthinen. Liebigs Ann Chem 957–960
Weinstein IB (1982) Carcinogenesis as a multistage process — Experimental evidence. In: Host factors in carcinogenesis. IARC Sci Publ 39:9–25
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Norpoth, K., Stücker, W., Krewet, E. et al. Biomonitoring of benzene exposure by trace analyses of phenylguanine. Int Arch Occup Environ Health 60, 163–168 (1988). https://doi.org/10.1007/BF00378692
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00378692