Summary
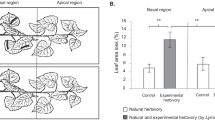
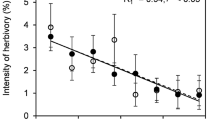
I compared the induced alkaloidal response in undamaged leaves of plants subjected to herbivory by the larvae of Manduca sexta and to different simulations of this herbivory; all herbivory treatments removed similar amounts of leaf mass. Although larval feeding induced a significant increase (2.2x) in alkaloid concentrations compared to undamaged plants, the alkaloid responses to larval feeding were significantly lower than the responses to an herbivory simulation (4x controls) which involved removing the same amount of leaf area from the same positions on the leaf, over a similar time period. Moreover, another herbivory simulation, identical in amount of leaf mass removed and duration of damage to the larval feeding, but without regard to spatial array of leaf damage, resulted in an alkaloidal response (5.5x controls) higher still than the previous herbivory simulation. In a second experiment the importance of leaf vein damage on the induced alkaloidal response was examined. Here, leaf removal that involved cutting leaf tissues from between secondary veins before removing the midrib, resulted in alkaloidal responses that were significantly lower (1.7x controls) than responses from leaf removal that involved cutting both veins and midribs along with the intervein tissues (2.6x controls). Vein damage alone did not produce a significant response. These results indicate that herbivory is difficult to simulate: that how a leaf is damaged can be as important as the magnitude of leaf damage in determining a plant's response to damage.
Similar content being viewed by others
References
Baldwin IT (1988a) Damage-induced alkaloids in tobacco: potbound plants are not inducible. J Chem Ecol 14:1113–1120
Baldwin IT (1988b) Short-term damage induced alkaloids protect plants. Oecologia 75:367–370
Baldwin IT (1988c) The mechanism of damage-induced alkaloids in wild tobacco. J Chem Ecol (in press)
Baldwin IT, Schultz JC (1983) Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science 221:277–279
Capinera JL, Roltsch WJ (1980) Response of wheat seedlings to actual and simulated migratory grasshopper defoliation. J Econ Entomol 73:258–261
Caswell H, Reed FC (1975) Indigestibility of C4 bundle sheath cells by the grasshopper Melanoplus confusus. Ann Entomol Soc Am 68:686–688
Caswell H, Reed FC (1976) Plant herbivory interactions. The indigestibility of C4 bundle sheath cells by grasshoppers. Oecologia (Berlin) 26:151–156
Chew V (1977) Comparison among treatment means in an analysis of variance. USDA Publication ARS/H
Hagen RH, Chabot JF (1986) Leaf anatomy of maples (Acer) and host use by Lepidoptera larvae. Oikos 47:335–345
Harvell CD (1986) The ecology and evolution of inducible defenses in a marine bryozoan: cues, costs and consequences. Am Nat 128:810–823
Haukioja E, Neuvonen S (1985) Induced long-term resistance of birch foliage against defoliators: defense or incidental? Ecology 66:1303–1308
Heinrich B (1971) The effect of leaf geometry on the feeding behavior of the caterpillar of Manduca sexta (Sphingidae). Anim Behav 19:119–127
Hori K (1976) Plant growth-regulating factor in the salivary gland of several heteropterous insects. Comp Biochem Physiol 53B:435–438
McFadden MW (1968) Observations on feeding and movement of tobacco hornworm larvae. J Econ Entomol 61:352–356
Madden AH (1945) Biology of the tobacco hornworm in the southern cigar-tobacco district. U.S.D.A. Tech Bull 896
Miles PW, Lloyd J (1967) Synthesis of a plant hormone by the salivary apparatus of plant-sucking Hemiptera. Nature 213:801–802
Rosa N (1973) Sampling of NIcotiana tabacum leaf lamina to surmount the problem of non-uniform distribution of total alkaloids. Can J Bot 51:289–291
Rossiter M, Schultz JC, Baldwin IT (1988) Relationships among defoliators, red oak phenolics, and gypsy moth growth and reproduction. Ecology 69:267–277
Ryan CA, Green TR (1974) Proteinase inhibitors in natural plant protection. Rec Adv Phytochem 18:123–140
Ryan TA Jr, Joiner BL, Ryan BF (1982) Minitab reference manual. Minitab Inc., State College, PA
Ryan CA, Bishop PD, Graham JS, Broadway RM, Duffey SS (1986) Plant and fungal cell wall fragments activate expression of proteinase inhibitor genes for plant defense. J Chem Ecol 12:1025–1036
Zeringue HJ (1987) Changes in cotton leaf chemistry induced by volatile elicitors. Phytochemistry 26:1357–1360
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baldwin, I.T. The alkaloidal responses of wild tobacco to real and simulated herbivory. Oecologia 77, 378–381 (1988). https://doi.org/10.1007/BF00378046
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00378046