Abstract
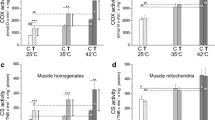
We investigated the effects of endurance training (20 m/min, 60 min/day, 5 days/week) on myosin heavy-chain (MHC) isoforms and succinic dehydrogenase (SDH) activity in rat crural and costal diaphragms, and plantaris muscles. Although the 4-week endurance training produced significant (P<0.05) increases, both in SDH activity and the percentage of isoform HCIIa in the plantaris of the trained rat compared with the sedentary control rat, these alterations did not occur in either the crural or costal diaphragms. After 10 weeks of endurance training, trained animals had significantly (P<0.05) higher SDH activity in the costal diaphragm and the plantaris. Moreover, a significant (P<0.05) decrease occurred in the percentage of HCIIb in the costal diaphragm, and a significant (P<0.01) decrease in the percentage of HCIIb concomitant with a significant (P<0.05) increase of HCIIa resulted in the plantaris. However, the crural diaphragm did not show any significant changes after 10 weeks of endurance training. These results indicate that endurance training induces an alteration in the expression of an MHC phenotype, in addition to causing an increase in oxidative enzyme activity. However, the alterations in response to endurance training are apparently not uniform, varying between regions and/or kinds of muscles.
Similar content being viewed by others
References
Akabas SR, Bazzy AR, DiMauro S, Haddad GG (1989) Metabolic and functional adaptation of the diaphragm to training with resistive loads. J Appl Physiol 66:529–535
Bär A, Pette D (1988) Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett 235:153–155
Billeter R, Heizmann CW, Howald H, Jenny E (1981) Analysis of myosin light and heavy chain types in single human skeletal muscle fibers. Eur J Biochem 116:389–395
Brooks GA, White TP (1978) Determination of metabolic and heart rate responses of rats to treadmill exercise. J Appl Physiol 45:1009–1015
Burke RE, Edgerton VR (1975) Motor unit properties and selective involvement in movement. Exercise Sport Sci Rev 3:31–81
Cooperstein SJ, Lazarow A, Kurfess NJ (1950) A microspectrophotometric method for the determination of succinic dehydrogenase. J Biol Chem 186:129–139
Crosfill ML, Widdicombe JG (1961) Physical characteristics of the chest and lungs and the work of breathing in different mammalian species. J Physiol (Lond) 158:1–14
DeTroyer A, Sampson M, Sigrist S, Macklem PT (1981) The diaphragm: two muscles. Science 213:237–238
Edwards RHT, Faulkner JA (1985) Structure and function of the respiratory muscles. In: Roussos C, Macklem PT (eds) The thorax, chapter 9, part A. Dekker, New York, pp 297–326
Farkas GA, Roussos C (1982) Adaptability of the hamster diaphragm to exercise and/or emphysema. J Appl Physiol 53:1263–1272
Fitzsimons DP, Diffee GM, Herrick RE, Baldwin KM (1990) Effects of endurance exercise on isomyosin patterns in fastand slow-twitch skeletal muscles. J Appl Physiol 68:1950–1955
Fregosi RF, Sanjak M, Paulson DJ (1987) Endurance training does not affect diaphragm mitochondrial respiration. Respir Physiol 67:225–237
Gollnick PD, Armstrong RB, Saltin B, Saubert CW IV, Sembrowich WL, Shepherd RE (1973) Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol 34:107–111
Günther PG (1953) Das muskuläre Substrat der Bewegungsund Halteleist-ung des menschlichen Zwerchfells. Acta Anat 17:348–352
Holloszy JO, Booth FW (1976) Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol 38:273–291
Keith A (1905) The nature of the mammalian diaphragm and pleural cavities. J Anat Physiol 39:243–284
Metzger JM, Scheidt KB, Fitts RH (1985) Histochemical and physiological characteristics of the rat diaphragm. J Appl Physiol 58:1085–1091
Monges H, Salducci J, Naudy B (1978) Dissociation between the electrical activity of the diaphragmatic dome and crural muscular fibers during esophageal distention, vomiting and eructation. An electromyographic study in the dog. J Physiol (Paris) 74:541–554
Moore RL, Gollnick PD (1982) Response of ventilatory muscles of the rat to endurance training. Pflügers Arch 392:268–271
Pette D, Müller W, Leisner E, Vrbová G (1976) Time dependent effects on contractile properties, fibre population, myosin light chains and enzymes energy metabolism in intermittently and continuously stimulated fast twitch muscles of the rabbit. Pflügers Arch 364:103–112
Powers SK, Lawler J, Criswell D, Dodd S, Grinton S, Bagby G, Silverman H (1990) Endurance-training-induced cellular adaptations in respiratory muscles. J Appl Physiol 68:2114–2118
Powers SK, Lawler J, Criswell D, Silverman H, Forster HV, Grinton S, Harkins D (1990) Regional metabolic differences in the rat diaphragm. J Appl Physiol 69:648–650
Sieck GC, Fournier M (1989) Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol 66:2539–2545
Sieck GC, Cheung T, Blanco CE (1991) Diaphragm capillarity and oxidative capacity during postnatal development. J Appl Physiol 70:103–111
Sugita H, Okumura Y, Ayai K (1969) Application of a property of troponin to determination of tropomyosin content of a small piece of muscle. J Biochem (Tokyo) 65:971–972
Sugiura T, Murakami N (1990) Separation of myosin heavy chain isoforms in rat skeletal muscles by gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biomed Res 11:87–91
Sugiura T, Morimoto A, Sakata Y, Watanabe T, Murakami N (1990) Myosin heavy chain isoform changes in rat diaphragm are induced by endurance training. Jpn J Physiol 40:759–763
Termin A, Staron RS, Pette D (1989) Myosin heavy chain isoforms in histochemically defined fiber types of rat muscle. Histochemistry 92:453–457
Termin A, Staron RS, Pette D (1989) Changes in myosin heavy chain isoforms during chronic low-frequency stimulation of rat fast hindlimb muscles. A single-fiber study. Eur J Biochem 186:749–754
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sugiura, T., Morimoto, A. & Murakami, N. Effects of endurance training on myosin heavy-chain isoforms and enzyme activity in the rat diaphragm. Pflügers Arch 421, 77–81 (1992). https://doi.org/10.1007/BF00374736
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00374736