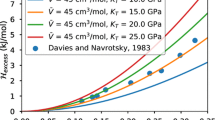
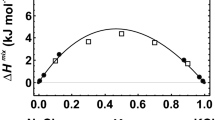
Abstract
The compositions of liquids coexisting with experimentally grown crystals of olivine, plagioclase, clinopyroxene, orthopyroxene, leucite, spinel, rhombohedral oxide, melilite and potassium feldspar are used to define, through mass action expressions of liquid/solid equilibrium, compositional derivatives of the Gibbs free energy of mixing of naturally occuring silicate liquids as a function of temperature, pressure and the fugacity of oxygen. The available experimental data describe these derivatives over a range of compositions which includes basic magmas. Therefore, for silicate liquids in this composition range, the topology of the Gibbs free energy of mixing can be approximated from experimental determinations of its derivatives. The majority of the existing thermodynamic data on the liquid phase is consistent with the application of regular solution theory to model the free energy of mixing. Strictly symmetric, temperature and pressure independent, regular solution interaction parameters are calibrated from this phase equilibrium data using regression techniques which have their basis in inverse theory. These techniques generate numerically stable interaction parameters which incorporate inter-variable correlation and account for experimental uncertainty. The regular solution model fits the available data on anhydrous silicate liquids to within the accuracy of the thermodynamic database +/−550 cals). Extensions to regular solution theory allow water solubility in more silica rich liquids to be modelled somewhat less accurately (+/−750 cals).
The topology of the excess free energy of mixing surface is strongly asymmetric, possessing a single multicomponent saddle point which defines a spinodal locus. Given this prediction of a multicomponent spinode, a mathematical procedure based upon minimisation of the Gibbs free energy of mixing is developed for the calculation of the compositions of coexisting immiscible liquids. Predicted binodal compositions substantially agree with elemental liquid/liquid partitioning trends observed in lavas. Calculations suggest that an immiscible dome, in temperature-composition space, intersects the liquidus field of the magma type tholeiite. Immiscible phenomena are predicted at sub-liquidus temperatures for the bulk compositions of more basic or alkalic lavas, but are absent in more siliceous rock types for temperatures of the metastable liquid down to 900 K.
The regular solution model is used in four petrological applications. The first concerns a prediction of the binary olivine-liquid phase diagram. The calculated geometry exhibits a minimum near Fa75, which, though not in accord with experimental results on the pseudobinary system, compares quite favorably with olivine-liquid phase equilibria interpreted from rhyolites, namely that the olivine phenocrysts of rhyolites are more iron rich than their coexisting liquids. The second petrological example concerns estimating the depth of the source regions of several basic lavas whose compositions cover a range from ugandite to basaltic andesite. The third application is a calculation of the saturation temperatures and compositions of plagioclase and olivine in four experimental basaltic liquids and a prediction of the liquidus temperatures and first phenocryst compositions of the Thingmuli lava series of Eastern Iceland. Lastly, enthalpies of fusion are computed for a variety of stoichiometric compounds of geologic interest. These demonstrate good agreement with calorimetrically measured quantities
Similar content being viewed by others
References
Adamkovicova K, Kosa L, Proks I (1980) The heat of fusion of calcium silicate. Inst Inorg Chem, Slovak Acad Sci Silikaty (Prague) 24:193–201
Anderson DJ, Lindsley DH (1981) A valid Margules formulation for an asymmetric ternary solution: revision of the olivine-ilmenite thermometer, with applications. Geochim Cosmochim Acta 45:847–854
Barron LM (1972) Thermodynamic multicomponent silicate equilibrium phase calculations. Am Mineral 57:809–823
Barron, L.M. (1978) The geometry of multicomponent exsolution. Am J Sci 278:1269–1306
Barron LM (1981) The calculated geometry of silicate liquid immiscibility. Geochim Cosmochim Acta 45:495–512
Bender JF, Hodges FN, Bence AE (1978) Petrogenesis of basalts from the project FAMOUS area: experimental study from 0 to 15 kbars. Earth Planet Sci Lett 41:277–302
Beswick AE, Carmichael ISE (1978) Constraints on Mantle source compositions imposed by phosphorus and the rare-earth elements. Contrib Mineral Petrol 67:317–330
Birch, F. (1966) Compressibility; elastic constants. In: Handbook of Physical Constants (SP Clark Jr, ed). Geol Soc Am Mem 97:97–173
Bottinga Y, Richet P (1978) Thermodynamics of liquid silicates; a preliminary report. Earth Planet Sci Lett 40:382–400
Bottinga Y, Weill DF, Richet P (1981) Thermodynamic modeling of silicate melts. In: Thermodynamics of Minerals and Melts (RC Newton, A Navrotsky, BJ Wood, eds) pp 207–245 New York: Springer-Verlag
Bowen NL (1913) The melting phenomena of the plagioclase feldspars. Am J Sci, 4th series 40:161–185
Bowen NL, Schairer JF (1935) The system, MgO-FeO-SiO2. Am J Sci 29:151–217
Bowen NL, Schairer JF, Posnjak E (1933) The system CaO-FeO-SiO2. Am Jour Sci 26:193–284
Brown FH (1971) Volcanic petrology of Recent volcanic rocks in the Lake Rudolf region, Kenya. Unpublished Ph. D. thesis, University of California, Berkeley
Brown FH, Carmichael ISE (1969) The Quaternary volcanoes of the Lake Rudolf region: I. The basanite-tephrite series of the Korath Range. Lithos 2:239–260
Brown FH, Carmichael ISE (1971) The Quaternary volcanoes of the Lake Rudolf region: II. The lavas of North Island, South Island, and the Barrier. Lithos 4:305–323
Burnham CW (1974) NaAlSi3O8-H2O solutions: a thermodynamic model for hydrous magmas. Bull Soc Fr Mineral Cristallogr 97:223–230
Burnham CW (1975a) Water and magmas; a mixing model. Geochim Cosmochim Acta 39:1077–1084
Burnham CW (1975b) Thermodynamics of melting in experimental silicate-volatile systems. Fortschr Miner 52:101–118
Burnham CW (1979) The importance of volatile constituents. In: The Evolution of the Igneous Rocks — Fiftieth Anniversary Perspectives (HS Yoder, Jr, ed) pp 439–482 New Jersey: Princeton University Press
Burnham CW (1981) The nature of multicomponent aluminosilicate melts. In: Chemistry and Geochemistry of Solutions at High Temperatures and Pressures (DT Rickard, FE Wickman, eds). Phys Chem Earth 13/14:197–229
Burnham CW, Jahns RH (1962) A method for determining the solubility of water in silicate liquids. Am J Sci 260:721–745
Carmichael ISE (1960) The pyroxenes and olivines from some Tertiary acid glasses. J Petrol 1:309–336
Carmichael ISE (1962) Pantelleritic liquids and their phenocrysts. Mineral Mag 33:86–113
Carmichael ISE (1964) The petrology of Thingmuli, a Tertiary volcano in eastern Iceland. J Petrol 5:435–460
Carmichael ISE (1967a) The iron-titanium oxides of silicic volcanic rocks and their associated ferromagnesian silicates. Contrib Mineral Petrol 14:36–64
Carmichael ISE (1967b) The mineralogy of Thingmuli, a Tertiary volcano in Eastern Iceland. Am Mineral 52:1815–1841
Carmichael ISE, Ghiorso MS (in prep) Intensive variables in siliceous magmas.
Carmichael ISE, Nicholls J, Spera FJ, Wood BJ, Nelson SA (1977) High-temperature properties of silicate liquids: applications to the equilibration and ascent of basic magma. Phil Trans R Soc Lond A 286:373431
Carstens H (1979) Liquid immiscibility in basic alkaline magmas. Chem Geol 27:297–307
Charlu TV, Newton RC, Kleppa OJ (1981) Thermochemistry of synthetic Ca2Al2SiO7 (gehlenite) — Ca2MgSi2O7 (akermanite) melilites. Geochim Cosmochim Acta 45:1609–1617
Chase MW Jr, Curnutt JL, McDonald RA, Syverud AN (1978) JANAF Thermochemical Tables, 1978 Supplement. J Phys Chem Ref Data 7:793–940
Chase MW, Curnutt JL, Prophet H, McDonald RA, Syverud AN (1975) JANAF Thermochemical Tables, 1975 Supplement. J Phys Chem Ref Data 4:1–175
Chase MW, Curnutt JL, Hu AT, Prophet H, Syverud AN, Walker LC (1974) JANAF Thermochemical Tables, 1974 Supplement. J Phys Chem Ref Data 3:311–480
Clark SP (1966) High Pressure Phase Equilibria. In: Handbook of Physical Constants (SP Clark Jr, ed.). Geol Soc Am Mem 97:345–370
Drake MJ (1972) The distribution of major and trace elements between plagioclase feldspar and magmatic silicate liquid: an experimental study. Unpublished Ph. D. thesis, University of Oregon
Eggler DH (1972) Water saturated and undersaturated melting relations in a Paricutin andesite and an estimate of water content in the natural magma. Contrib Mineral Petrol 34:261–271
Flanigan FJ, Kazdan JL (1971) Calculus Two: Linear and Nonlinear Functions, pp 443. New Jersey: Prentice-Hall, Inc.
French WJ (1971) The correlation between “Anhydrous” Crystallization temperatures and rock composition. Contrib Mineral Petrol 31:154–158
French WJ, Cameron EP (1981) Calculation of the temperatures of crystallization of silicates from basaltic melts. Mineral Mag 44:19–26
Gaskell DR (1982) The densities and structures of silicate melts. In: Advances in Physical Geochemistry (Vol. II) (S.K. Saxena, ed) pp 153–171 New York: Springer-Verlag
Ghiorso MS (1983) LSEQIEQ: A FORTRAN IV subroutine package for the analysis of multiple linear regression problems with possibly deficient pseudorank and linear equality and inequality constraints. Computers and Geosciences 9:391–416
Ghiorso MS, Carmichael ISE (1980) A regular solution model for met-aluminous silicate liquids: Applications to geothermometry, immiscibility, and the source regions of basic magma. Contrib Mineral Petrol 71:323–342
Ghiorso MS, Carmichael ISE Density calculations for silicate liquids. I. Revised method for aluminosilicate compositions by Bottinga, Weill, and Richet: A commentary, Geochim Cosmochim Acta (submitted)
Ghiorso MS, Carmichael ISE, Moret LK (1979) Inverted hightemperature quartz. Unit cell parameters and properties of the α-β inversion. Contrib Mineral Petrol 68:307–323
Gill PE, Murray W (eds) (1974) Numerical methods for constrained optimization, pp 283. New York: Academic Press Inc.
Graham A (1981) Kronecker Products and Matrix Calculus with Applications, pp 130. New York: John Wiley and Sons
Green DH, Hibberson WO, Jaques AL (1979) Petrogenesis of midocean ridge basalts. In: The Earth: Its Origin, Structure and Evolution (MW McElhinny ed.) Acad Press
Grove TL, Bryan WB (1983) Fractionation of pyroxene-phyric MORB at low pressures: An experimental study. Contrib Mineral Petrol (in press)
Grove TL, Gerlach DC, Sando TW (1982) Origin of calc-alkaline series lavas at Medicine Lake volcano by fractionation, assimilation and mixing. Contrib Mineral Petrol 80:160–182
Hamilton DL, Burnham CW, Osborn EF (1964) The solubility of water and effects of oxygen fugacity and water content on crystallization in mafic magmas. J Petrol 5:21–39
Helgeson HC, Delaney JM, Nesbitt HW, Bird DK (1978) Summary and critique of the thermodynamic properties of rock-forming minerals. Am J Sci 278–229
Helz RT (1973) Phase relations of basalts in their melting range at P144-01-5 kbar as a function of oxygen fugacity. Part I. Mafic Phases. J Petrol 14:249–302
Henry DJ, Navrotsky A, Zimmerman HD (1982) Thermodynamics of plagioclase melt equilibria in the system albite-anorthite-diopside. Geochim Cosmochim Acta 46:381–391
Hess PC (1971) Polymer model of silicate melts. Geochim Cosmochim Acta 35:289–306
Hess PC (1980) Polymerization model for silicate melts. In: Physics of Magmatic Processes (RB Hargraves, ed) pp 3–48 Princeton, New Jersey: Princeton Univ Press
Hildreth EW (1977) The magma chamber of the Bishop Tuff: gradients in temperature, pressure, and composition. Unpublished Ph. D. thesis, University of California, Berkeley
Hill WL, Faust GT, Reynolds DS (1944) The binary system P2O5-2 CaO · P2O5. Am J Sci, 242 (part I), 457–477
Hon R, Henry DJ, Navrotsky A, Weill DF (1981) A thermochemical calculation of the pyroxene saturation surface in the system diopside-albite-anorthite. Geochim Cosmochim Acta 45:157–161
Hostetler CJ, Drake MJ (1980) Predicting major element mineral/melt equilibria: A statistical approach. J Geophys Res 85:3789–3796
Kelley KK (1960) Contributions to the data on theoretical metallurgy: part 13, High temperature heat content, heat capacity and entropy data for the elements and inorganic compounds. US Bur Mines Bull 584, pp 232
Kerrick DM, Darken LS (1975) Statistical thermodynamic models for ideal oxide and silicate solid solutions, with applications to plagioclase. Geochim Cosmochim Acta 39:1431–1442
Khitarov NI, Kadik AA (1973) Water and carbon dioxide in magmatic melts and peculiarities of the melting process. Contrib Mineral Petrol 41:205–215
Khitarov NI, Lebedev EB, Regartan EV, Arseneva RV (1959) Solubility of water in basaltic and granitic melts. Geochemistry 5:479–492
King EG, Orr RL, Bonnickson KR (1954) Low temperature heat capacity, entropy at 298.16° K and high temperature heat content of sphene (CaTiSiO5). J Am Chem 76:4320–4321
Kosa L, Adamkovicova K, Proks I (1981) Determining the heat of incongruent decomposition of merwinite. Inst Inorg Chem, Slovak Acad Sci, Silikaty (Prague) 25(3):199–208
Lawson CL, Hanson RJ (1974) Solving Least Squares Problems, pp 340. New Jersey: Prentice-Hall Inc.
Leeman WP (1974) Experimental determination of partitioning of divalent cations between olivine and basaltic liquid. Part II. Unpublished Ph. D. thesis, University of Oregon
Longhi J, Walker D, Hays JF (1978) The distribution of Fe and Mg between olivine and lunar basaltic liquid. Geochim Cosmochim Acta 42:1545–1558
Luhr JF, Carmichael ISE (1980) The Colima Volcanic Complex, Mexico. I. Post-Caldera andesites from Volcán Colima. Contrib Mineral Petrol 71:343–372
Mahood GA (1981) Chemical evolution of a Pleistocene rhyolitic center: Sierra La Primavera, Jalisco, Mexico. Contrib Mineral Petrol 77:129–149
Massen CR (1968) Ionic equilibria in liquid silicates. J Am Ceram Soc, 51:134–143
Meijering JL (1950) Segregation in regular ternary solutions. Part I. Philips Res Rep 5:333–356
Meijering JL (1951) Segregation in regular ternary solutions. Part II. Philips Res Rep 6:183–210
Meyer HOA, Boyd FR (1972) Composition and origin of crystalline inclusions in natural diamonds. Geochim Cosmochim Acta 36:1255–1273
Mo X, Carmichael ISE, Rivers M, Stebbins J (1982) The partial molar volume of Fe2O3 in multicomponent silicate liquids and the pressure dependence of oxygen fugacity in magmas. Mineral Mag 45:237–245
Morse SA (1980) Basalts and Phase Diagrams, pp 493. Heidelberg: Springer-Verlag
Mukherjee A, Bhattacharya A (1980) High-pressure silicate liquidus data: Retrieval of partial molar enthalpy and partial molar entropy of mixing, a test of the regular solution formulation, and comments on the P-T modelling of ascending magmas. Earth Planet Sci Lett 49:231–236
Mysen BO, Kushiro I (1977) Compositional variations of coexisting phases with degree of melting of peridotite in the upper mantle. Am Mineral 62:843–856
Mysen BO, Virgo D, Seifert FA (1982) The structure of silicate melts: Implications for chemical and physical properties of natural magma. Rev Geophys and Space Phys 20:353–383
Nelson SA, Carmichael ISE (1979) Partial molar volumes of oxide components in silicate liquids. Contrib Mineral Petrol 71:117–124
Nesbitt HW, Fleet ME (1981) An ion-association model for PbO-SiO2 melts: Interpretation of thermochemical, conductivity, and density data. Geochim Cosmochim Acta 45:235–244
Newton KC (1976) Thermochemistry of garnets and aluminous pyroxenes in the CMAS system. In: Thermodynamics in Geology (DC Fraser, ed), pp 29–56. Holland: D Reidel 1976
Newton RC, Charlu TV, Kleppa OJ (1980) Thermochemistry of the high structural state plagioclases. Geochim Cosmochim Acta 44:933–941
Nicholls J (1976) The activities of components in natural silicate melts. In: Thermodynamics in Geology (DC Fraser, ed), pp 327–348. Holland: D Reidel
Nicholls J (1980) A simple thermodynamic model for estimating the solubility of H2O in magmas. Contrib Mineral Petrol 74:211–220
Nicholls J, Carmichael ISE (1972) The equilibrium temperature and pressure of various lava types with spinel- and garnetperidotite. Am Mineral 57:941–959
Nisbet EG, Bickle MJ, Martin A (1977) The mafic and ultramafic lavas of the Belingwe Greenstone Belt, Rhodesia. J Petrol 18:521–566
Orr RL (1953) High temperature heat contents of magnesium orthosilicate and ferrous orthosilicate. J Am Chem Soc 75:528–529
Oxtoby S, Hamilton DL Calculation of the solubility of water in granitic melts. In: Progress in Experimental Petrology (W.S. Mackenzie ed) Natural Environment Research Council, Great Britain, 4th Progress Report, 37–40
Parlett BN (1980) The Symmetric Eigenvalue Problem, pp 348. New Jersey: Prentice-Hall, Inc
Philpotts AR (1976) Silicate liquid immiscibility: its probable extent and petrogenetic significance. Am J Sci 276:1147–1177
Philpotts AR (1979) Silicate liquid immiscibility in tholeiitic basalts. J Petrol 20:99–118
Philpotts AR (1982) Compositions of immiscible liquids in volcanic rocks. Contrib Mineral Petrol 80:201–218
Pitzer KS (1981) Characteristics of very concentrated aqueous solutions. In: Chemistry and Geochemistry of solutions at High Temperatures and Pressures (Rickard DT, Wickman FE, eds) Phys Chem Earth 13/14:249–272
Rammensee W, Fraser DG (1982) Determination of activities in silicate melts by Knudsen cell mass spectrometry — I. The system NaAlSi3O8-KAlSi3O8. Geochim Cosmochim Acta 46:2269–2278
Reed MH (1982) Calculation of multicomponent chemical equilibria and reaction processes in systems involving minerals, gases and an aqueous phase. Geochim Cosmochim Acta 46:513–528
Rivers ML, Ghiorso MS (1980) Free energy minimization in multicomponent systems: Applications to silicate liquid immiscibility. GSA Abstr with Progr 12: no. 7, 511
Robie RA, Hemingway BS, Fisher JR (1978) Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 Pascals) pressure and at higher temperatures. US Geol Surv Bull 1452:pp 456
Roedder E (1979) Silicate liquid immiscibility in magmas. In: The Evolution of the Igneous Rocks-Fiftieth Anniversary Perspectives (Yoder HS, ed), pp 15–58. New Jersey: Princeton University Press
Roeder PL (1974) Activity of iron and olivine solubility in basaltic liquids. Earth Planet Sci Lett 23:397–410
Sack RO (1980) Some constraints on the thermodynamic mixing properties of Fe-Mg orthopyroxenes and olivines. Contrib Mineral Petrol 71:257–269
Sack RO (1982a) Spinels as petrogenetic indicators: activity-composition relations at low pressures. Contrib Mineral Petrol 79:169–186
Sack RO (1982b) Fe2-Mg2 and TiAl2-MgSi2 exchange rate between clinopyroxenes and silicate melts. GSA Abstr with Progr 14:no. 7, 606
Sack RO, Carmichael ISE, Rivers M, Ghiorso MS (1981) Ferricferrous equilibria in natural silicate liquids at 1 bar. Contrib Mineral Petrol 75:369–376
Skinner BJ (1966) Thermal Expansion. In: Handbook of Physical Constants (Clark SP, ed) Geol Soc Am Mem 97:75–96
Smith MP (1983) A feldspar-liquid geothermometer. Geophys Res Lett 10:193–195
Stebbins JF, Carmichael ISE (1981a) Heats fusion of diopside and sanidine and high pressure volume changes in aluminous silicate liquids. GSA Abstr with Progr 13:no 7, 560
Stebbins JF, Carmichael ISE (1981b) The heat of fusion of fayalite: assessment of oxidation in calorimetric measurements. EOS, 62: no. 45, 1070
Stebbins JF, Carmichael ISE, Weill DF (1980) High temperature heat contents, heat capacities, and solution properties of plagioclase composition liquids. GSA Abstr with Progr 12, no. 7, 528
Stolper E (1980) A phase diagram for mid-ocean ridge basalts: Preliminary results and implications for petrogenesis. Contrib Mineral Petrol 74:13–27
Stolper E (1982a) Water in silicate glasses: an infrared spectroscopic study. Contrib Mineral Petrol 81:1–17
Stolper E (1982b) On the speciation of water in silicate melts. Geochim Cosmochim Acta 46:2609–2620
Stull DR, Prophet H (1971) JANAF Thermochemical Tables, 2nd ed. Nat Stand Ref Data Ser, Nat Bur Stand (US), 37: pp. 1141
Takahashi E (1978) Partitioning of Ni+2, Co+2, Fe+2, Mn+2, and Mg+2 between olivine and silicate melts: compositional dependence of partition coefficient. Geochim Cosmochim Acta 42:1829–1844
Takahashi E (1980) Melting relations of an alkali-olivine basalt to 30 kbars, and their bearing on the origin of alkali basalt magmas. Carnegie Inst Wash Ann Yearb 79:271–276
Thompson RN (1974) Primary basalts and magma genesis I. Contrib Mineral Petrol 45:317–341
Thompson RN (1975) Primary basalts and magma genesis II. Contrib Mineral Petrol 52:213–232
Toop GW, Samis CS (1962) Activities of ions in silicate melts. Trans Metall Soc AIME, 224:878–887
Visser W, Koster van Groos AF (1979) Effects of P2O5 and TiO2 on liquid-liquid equilibria in the system K2O-FeO-A12O3-SiO2. Am J Sci 279:970–988
Walker D, Mullins O Jr (1981) Surface tension of natural silicate melts from 1,200°–1,500° C and implications for melt structure. Contrib Mineral Petrol 76:455–462
Walker D, Shibata T, Delong SE (1979) Abyssal tholeiites from the oceanographer fracture zone. II. Phase equilibria and mixing. Contrib Mineral Petrol 70:111–125
Weill DF, Hon R, Navrotsky A (1980a) The igneous system CaMgSi2O6-CaAlSi2O8-NaAlSi3O8: variations on a classic theme by Bowen. In: Physics of Magmatic Processes (RB Hargraves, ed), pp 49–92. New Jersey: Princeton University Press
Weill DF, Stebbins JF, Hon R, Carmichael ISE (1980b) The enthalpy of fusion of anorthite. Contrib. Mineral. Petrol. 74, 95–102
Wood BJ (1976) Experimental determination of mixing properties of solid solutions with particular reference to garnet and clinopyroxene solutions. In: Thermodynamics in Geology, (Fraser DC, ed), pp 11–28. Holland: D Reidel
Wood BJ, Kleppa OJ (1981) Thermochemistry of forsterite-fayalite olivine solutions. Geochim Cosmochim Acta 45:529–534
Wood MI, Hess PC (1980) The structural role of A12O3 and TiO2 in immiscible liquids in the system SiO2-MgO-CaO-FeO-TiO2-Al2O3. Contrib Mineral Petrol 72:319–328
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ghiorso, M.S., Carmichael, I.S.E., Rivers, M.L. et al. The Gibbs free energy of mixing of natural silicate liquids; an expanded regular solution approximation for the calculation of magmatic intensive variables. Contr. Mineral. and Petrol. 84, 107–145 (1983). https://doi.org/10.1007/BF00371280
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00371280