Abstract
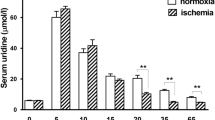
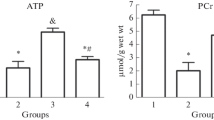
Uric acid (UA) is released from the heart of many species, including man, and its site of formation has been shown to be the microvascular endothelium. Since UA reacts with oxygen radicals in vitro, experiments were conducted on guinea pig hearts perfused with Krebs-Henseleit buffer (KHB) to evaluate whether the formation of UA could afford protection from damage by radicals and oxidants. The following results were obtained: (1) Upon addition of the hydroxyl radical scavenger DMSO to the perfusate, the coronary rate of release of endogenous uric acid was increased relative to the precursor purines. (2) UA was degraded during passage through the coronary system and also in KHB in vitro after addition of substances generating hydroxyl radicals or hypochlorite. Superoxide (O −2 ) radicals did not seem to react directly with UA, though UA concentration-dependently quenched the chemiluminescence generated from luminol in the presence of O −2 and OH radicals. (3) Coronary dilatation by acetylcholine (Ach) and sub-μM concentrations of adenosine, induced by both via endothelial mechanisms, was attenuated after prolonged inhibition of endothelial UA formation by allopurinol. Furthermore, the effect of Ach but not of adenosine proved acutely sensitive to methylene blue and O −2 , substances known to inactivate EDRF. This finding suggests involvement of EDRF in Ach-mediated, but not in adenosine-induced dilatation of the intact coronary system. Exogenously applied UA prevented the impairment of vascular responses to Ach and adenosine caused by allopurinol, and to Ach upon generation of O −2 .(4) Hearts performed more pressure-volume work and exhibited greater functional stability when perfused with KHB supplemented with UA in a physiological concentration. It is concluded that uric acid can actually serve as a physiologic radical scavenger and antioxidant, maintaining functional responsiveness of the coronary system and of the myocardium.
Similar content being viewed by others
References
Achterberg PW, Stroeve RJ, De Jong JW (1986) Myocardial adenosine cycling rates during normoxia and under conditions of stimulated purine release. Biochem J 235:13–17
Ames BN, Cathcart R, Schwiers E, Hochstein P (1981) Uric acid provides an antioxidant defense in humans against oxidantand radical-caused aging and cancer: A hypothesis. Proc Natl Acad Sci USA 78:6858–6862
Becker BF, Gerlach E (1984) Acute effects of nicotine on hemodynamic and metabolic parameters of isolated, perfused hearts of guinea pigs and rats. Klin Wochenschr 62 (Suppl II):58–66
Becker BF, Gerlach E (1987) Uric acid, the major catabolite of cardiac adenine nucleotides and adenosine, originates in the coronary endothelium. In: Gerlach E, Becker BF (eds) Topics and perspectives in adenosine research. Springer, Berlin Heidelberg New York, pp 209–222
Becker BF, Permanetter B, Sebening H, Blömer H, Gerlach E (1987) Uric acid in heart patients: Cardiac production and disappearance in the lung. Pflügers Arch 408 (Suppl I): R14
Becker BF, Özçelik T, Reinholz N, Leipert B, Gerlach E (1988) Oxygen radical scavenging, a possible physiologic function of uric acid in the coronary system. Pflügers Arch 411 (Suppl I):R26
Betts WH (1985) Detecting oxy radicals by chemiluminescence. In: Greenwald RA (ed) CRC Handbook of methods for oxygen radical research. CRC Press, Boca Raton, Florida, pp 197–201
Cadenas E, Sies H (1984) Low-level chemiluminescence as an indicator of singlet molecular oxygen in biological systems. Methods Enzymol 105:221–231
Cohen G (1985) The Fenton reaction. In: Greenwald RA (ed) CRC Handbook of methods for oxygen radical research. CRC Press, Boca Raton, Florida, pp 55–64
Das DK, Engelman RM, Clement R, Otani H, Prasad MR, Rao PS (1987) Role of xanthine oxidase inhibitor as free radical scavenger: A novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem Biophys Res Commun 148:314–319
Deby C, Deby-Dupont G, Noel F-X, Lavergne L (1981) In vitro and in vivo arachidonic acid conversions into biologically active derivatives are enhanced by uric acid. Biochem Pharmacol 30:2243–2249
Dobos D (1975) Electrochemical data. Elsevier, Amsterdam, pp 250–261
Freeman BA, Crapo JD (1982) Biology of disease. Free radicals and tissue injury. Lab Invest 47:412–426
Gerlach E, Nees S, Becker BF (1985) The vascular endothelium: A survey of some newly evolving biochemical and physiological features. Basic Res Cardiol 80:459–474
Grootveld M, Halliwell B (1987) Measurement of allantoin and uric acid in human body fluids. A potential index of free radical reaction in vivo? Biochem J 243:803–808
Gryglewski RJ, Palmer RMJ, Moncada S (1986) Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320:454–456
Hodgson EK, Fridovich I (1976) The mechanism of the activity-dependent luminescence of xanthine oxidase. Arch Biochem Biophys 172:202–205
Howell RR, Wyngaarden JB (1960) On the mechanism of peroxidation of uric acid by hemoproteins. J Biol Chem 235: 3544–3550
Jackson CV, Mickelson JK, Stringer K, Rao PS, Lucchesi BR (1986) Electrolysis-induced myocardial dysfunction. A novel method for the study of free radical mediated tissue injury. J Pharmacol Meth 15:305–320
Jarasch E-D, Grund C, Bruder G, Heid HW, Keenan TW, Franke WW (1981) Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell 25:67–82
Komori K, Lorenz RR, Vanhoutte PM (1988) Nitric oxide, Ach and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol 255: H207-H212
Lucchesi BR, Mickelson JK, Homeister JW, Jackson CV (1987) Interaction of the formed elements of blood with the coronary vasculature in vivo. Fed Proc 46:63–72
Maples KR, Mason RP (1988) Free radical metabolite of uric acid. J Biol Chem 263:1709–1712
Martin W, Villani GM, Jothianandan D, Furchgott RF (1985) Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther 232:708–716
McCord JM (1987) Oxygen-derived radicals: A link between reperfusion injury and inflammation. Fed Proc 46:2402–2406
Nees S, Herzog V, Becker BF, Böck M, Des Rosiers Ch, Gerlach E (1985) The coronary endothelium: A highly active metabolic barrier for adenosine. Basic Res Cardiol 80:515–529
Ogino N, Yamamoto S, Hayaishi O, Tokkuyama T (1979) Isolation of an activator for prostaglandin hydroperoxidase from bovine vesicular gland cytosol and its identification as uric acid. Biochem Biophys Res Commun 87:184–191
Orowan E (1955) The origin of man. Nature 175:683–684
Palmer RMJ, Ferrige AG, Moncada S (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524–526
Pincemail J, Deby C, Thirion A, Dethier A, Deby-Dupont G, Goutier R (1986) Stimulation of cyclooxygenase by activated human neutrophils is enhanced by uric acid. Prostaglandins 32:101–105
Repine JE, Eaton JW, Anders MW, Hoidal JR, Fox RB (1979) Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. J Clin Invest 64:1642–1651
Rubanyi G, Vanhoutte PM (1985) Endothelium-removal decreases relaxations of canine coronary arteries caused by β-adrenergic agonists and adenosine. J Cardiovasc Pharmacol 7:139–144
Rubanyi GM, Vanhoutte PM (1986) Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol 250:H822-H827
Salin ML, McCord JM (1974) Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest 1005–1009
Spector T (1977) Inhibition of urate production by allopurinol. Biochem Pharmacol 26:355–358
Stewart DJ, Pohl U, Dézsi L, Bassenge E (1987) The role of endothelium-derived relaxing factor (EDRF) in the acetylcholine-induced dilation of coronary resistance vessels. Pflügers Arch 408 (Suppl I):R22
Usuda N, Reddy MK, Hashimoto T, Rao MS, Reddy JK (1988) Tissue specificity and species differences in the distribution of urate oxidase in peroxisomes. Lab Invest 58:100–111
Warren JS, Ward PA (1986) Review: Oxidative injury to the vascular endothelium. Am J Med Sci 292:97–103
Wayner DDM, Burton GW, Ingold KU, Barclay LRC, Locke SJ (1987) The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta 924:408–419
Zimmerman BJ, Parks DA, Grisham MB, Granger DN (1988) Allopurinol does not enhance antioxidant properties of extracellular fluid. Am J Physiol 255: H202-H206
Author information
Authors and Affiliations
Additional information
A preliminary report has been given at the Joint Meeting of the Deutsche Physiologische Gesellschaft and the Physiological Society, 16–19 March 1988, in Würzburg, Germany [6]
Rights and permissions
About this article
Cite this article
Becker, B.F., Reinholz, N., Özçelik, T. et al. Uric acid as radical scavenger and antioxidant in the heart. Pflügers Arch 415, 127–135 (1989). https://doi.org/10.1007/BF00370582
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00370582