Abstract
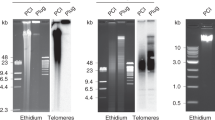
A fraction of DNA fragments of highly purified and completely unfolded eukaryotic DNA inevitably remains associated with chemically resistant nonhistone DNA-polypeptide complexes. This fraction can be isolated by nitrocellulose filtration because the polypeptide-associated DNA fragments are retained on nitrocellulose filters while bulk DNA passes through the filters. The fraction of AluI-fragmented DNA from human placenta retained on filters as a result of the binding factors (R-DNA, ∼12%) represents a subset of genomic sequences with a sequence complexity different from unfractionated DNA and DNA recovered in the filtrate (F-DNA). DNA sequences prevalent in the retained fraction were detected by differential plaque hybridization of a recombinant λgt10 library with radiolabeled F- and R-DNA fractions. Several recombinant phages showing much stronger hybridization signals with the R-DNA probe than with the F-DNA probe were selected, plaque-purified and analyzed. Analysis of the inserts of such clones showed that repetitive DNA sequences of the alphoid dimeric and tetrameric family, satellite III and satellite III-like sequences are highly enriched in the retained fraction, which indicates that these sequences specifically attract the polypeptides involved in the tightly bound and resistant complexes. This property of repetitive sequences is of interest since tandemly repetitive sequences have been suggested to code for locus-specific fixation and stabilization of the chromatin fiber in the cell nucleus.
Similar content being viewed by others
References
Baldini A, Smith DI, Rocchi M, Miller OJ, Miller DA (1989) A human alphoid DNA clone from the EcoRI dimeric family: Genomic and internal organization and chromosomal assignment. Genomics 5:822–828
Basler J, Hastie ND, Pietras D, Matsui SI, Sandberg AA, Berezney R (1981) Hybridization of nuclear matrix attached deoxyribonucleic acid fragments. Biochemistry 20:6921–6929
Berezney R (1984) Organizations and functions of the nuclear matrix. In: Hnilica LS (ed) Chromosomal nonhistone proteins, vol 4. CRC Press, Boca Raton, pp 119–180
Berezney R, Coffey DS (1976) The nuclear protein matrix: isolation, structure and function. Adv Enzyme Regul 14: 63–100
Beridze T (1982) Satellite DNA. Springer, Berlin Heidelberg New York
Blin N, Stafford DW (1976) Isolation of high molecular weight DNA. Nucleic Acids Res 3: 2303–2308
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81: 1991–1995
Ciejek EM, Tsai MJ, O'Malley BW (1983) Actively transcribed genes are associated with the nuclear matrix. Nature 306: 607–609
Cook PR, Brazell IA (1975) Supercoils in human DNA. J Cell Sci 19: 261–279
Feinberg AP, Vogelstein B (1984) A technique for radiolabeling DNA restriction nuclease fragments to high specific activity. Anal Biochem 132: 6–13
Frommer M, Prosser J, Tkachuk D, Reisner AH, Vincent PC (1982) Simple repeated sequences in human satellite DNA. Nucleic Acids Res 10: 547–563
Gasser SM (1988) Nuclear scaffold and the higher-order folding of eukaryotic DNA. In: Kahl G (ed) Architecture of eukaryotic genes. VCH, Weinheim, pp 461–471
Goldberg GI, Collier I, Cassel A (1983) Specific DNA sequences associated with the nuclear matrix in synchronized mouse 3T3 cells. Proc Natl Acad Sci USA 80: 6887–6891
Gross-Bellard M, Oudet P, Chambon P (1973) Isolation of high molecular weight DNA from mammalian cells. Eur J Biochem 36: 32–38
Henninghausen L, Lubon H (1987) Interaction of protein with DNA in vitro. Methods Enzymol 152: 721–735
Jackson DA, Cook PR, Patel SB (1984) Attachment of repeated sequences to the nuclear cage. Nucleic Acids Res 12: 6709–6726
Juodka B, Pfütz M, Werner D (1991) Chemical and enzymatic analysis of covalent bonds between peptides and chromosomal DNA. Nucleic Acids Res 19: 6391–6398
Käs E, Chasin LA (1987) Anchorage of the Chinese hamster dihydrofolate reductase gene to the nuclear scaffold occurs at intragenic regions. J Mol Biol 198: 677–692
Lu X, Werner D (1988) Construction and quality of cDNA libraries from cytoplasmic RNA not enriched in poly(A+). Gene 71: 157–164
Maio JJ (1971) DNA strand reassociation and polyribonucleotide binding in the African green monkley. J Mol Biol 56: 579–595
Manuelidis L (1978) Chromosomal localization of complex and simple repeated human DNAs. Chromosoma 66: 23–32
Matsumoto LH (1981) Enrichment of satellite DNA on the nuclear matrix of bovine cells. Nature 294: 481–482
Mirkovitch J, Mirault ME, Laemmli UK (1984) Organization of the higher order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell 39: 223–232
Musich PR, Brown FL, Maio JJ (1980) Highly repetitive component α and related alphoid DNAs in man and monkey. Chromosoma 80: 331–348
Neuer B, Werner D (1985) Screening of isolated DNA for sequences released from anchorage sites in nuclear matrix. J Mol Biol 181: 15–25
Neuer B, Plagens U, Werner D (1983) Phosphodiester bonds between polypeptides and chromosomal DNA. J Mol Biol 164: 213–235
Neuer-Nitsche B, Werner D (1987) Sub-set characteristics of DNA sequences involved in tight DNA/polypeptide complexes and their homology to nuclear matrix DNA. Biochem Biophys Res Commun 147: 335–339
Neuer-Nitsche B, Lu X, Werner D (1988) Functional role of a highly repetitive DNA sequence in anchorage of the mouse genome. Nucleic Acids Res 16: 8351–8360
Paulson JR, Laemmli UK (1977) The structure of histone-depleted metaphase chromosomes. Cell 12: 817–828
Robinson SJ, Nmall D, Idzerda R, McKnight GS, Vogelstein B (1983) The association of transcriptionally active genes with the nuclear matrix of the chicken oviduct. Nucleic Acids Res 11: 5113–5130
Robinson SJ, Nelkin BD, Vogelstein B (1984) The ovalbumin gene is associated with the nuclear matrix of chiken oviduct cells. Cell 28: 99–106
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Strauss F, Varshavsky A (1984) A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell 37: 889–901
Vissel B, Choo KH (1987) Human alpha satellite DNA-consensus sequence and conserved regions. Nucleic Acids Res 15: 6751
Vogelstein B, Pardoll DM, Coffey DS (1980) Supercoiled loops and DNA replication. Cell 22: 79–85
Vogt P (1990) Potential genetic functions of tandem repeated DNA sequence blocks in the human genome are based on a highly conserved ‘chromatin folding code’. Hum Genet 84: 301–336
Werner D, Neuer-Nitsche B (1989a) Discontinuities of peptide nature in DNA. In: Bekhor I, Liew CC (eds) Progress in nonhistone protein research, vol 3, CRC Press, Boca, Raton, pp 99–118
Werner D, Neuer-Nitsche B (1989b) Site-specific location of covalent DNA-polypeptide complexes in the chicken genome. Nucleic Acids Res 17: 6005–6015
Werner D, Rest R (1987) Radiolabelling of DNA/polypeptide complexes in isolated bulk DNA and in nuclear matrix DNA. Biochem Biophys Res Commun 147: 340–345
Werner D, Chanpu S, Müller M, Spiess E, Plagens U (1984) Antibodies to the most tightly bound proteins in DNA. Formation of immuno-complexes with nuclear matrix components. Exp Cell Res 51: 384–395
Werner D, Rest R, Neuer-Nitsche B (1988) Nuclear matrix. In: Kahl G (ed) Architecture of eukaryotic genes. VCH, Weinheim, pp 449–459
Willard HF (1985) Chromosome-specific organization of human alpha satellite DNA. Am J Hum Genet 37: 524–532
Willard HF, Waye JS (1987) Hierarchical order in chromosome-specific human alpha satellite DNA. Trends Genet 3: 192–198
Wu JC, Manuelidis L (1980) Sequence definition and organization of a human repeated DNA. J Mol Biol 142: 363–386
Author information
Authors and Affiliations
Additional information
by L. Manuelidis
This work contains parts of the Ph.D. thesis of M.P. (University of Giessen).
Rights and permissions
About this article
Cite this article
Pfütz, M., Gileadi, O. & Werner, D. Identification of human satellite DNA sequences associated with chemically resistant nonhistone polypeptide adducts. Chromosoma 101, 609–617 (1992). https://doi.org/10.1007/BF00360538
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00360538