Abstract
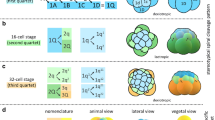
In this study we reinvestigate the early development of the freshwater mussel Dreissena polymorpha, previously studied by Meisenheimer (1901). The data include video time-lapse recordings of living embryos and bisbenzimide stains of fixed embryos as well as morphometry on fixed, serially-sectioned embryos. We present the cell lineage and cell cycle durations up to the first indication of symmetrization within this embryo. We show that early cell cycles last approximately 1h. A dramatic extension of cell cycle duration and a concomitant asynchrony among the various cell lines was observed starting at the fifth cleavage. Short cell cycles, like those of early blastomeres, were a constant property of the largest descendants of the 2d-cell line only. In contrast to Meisenheimer's observations and our experiences with other spiralian embryos, the cleavage pattern proved to follow multiple alternatives. The embryonic quadrants A-D were arranged in either a clockwise or counter-clockwise fashion and the chirality of the third cleavage was either dextral or sinistral irrespective of the arrangement of the quadrants. As a consequence, four different blastomere configurations were encountered and the dorsoventral axis could take four different angles with respect to the plane of first cleavage. The dorsal side was most easily recognized by the position of the 2d-micromere at the 16-cell stage. The fact that all of such embryos could develop into normal, uniform larvae is interpreted as the result of cell-cell interactions in morphogenetic regulation.
Similar content being viewed by others
References
Andéol Y (1994) Early transcription in different animal species: implication for transition from maternal to zygotic control in development. Roux's Arch Dev Biol 204:3–10
Atkinson JW (1971) Organogenesis in normal and lobeless embryos of the marine prosobranch Ilyanassa obsoleta. J Morphol 133:339–352
Cather JN, Verdonk NH (1974) The development of Bithynia tentaculata (Prosobranchia, Gastropoda) after removal of the polar lobe. J Embryol Exp Morphol 31:415–422
Clement AC (1952) Experimental studies on germinal localization in Ilyanassa. J Exp Zool 121:593–625
Crampton HE (1894) Reversal of cleavage in a sinistral gastropod. Ann NY Acad Sci 8:167–170
van Dam WI, Dohmen MR, Verdonk NH (1982) Localization of morphogenetic determinants in a special cytoplasm present in the polar lobe of Bithynia tentaculata (Gastropoda). W Roux' Arch 191:371–377
van Dongen CAM (1976) The development of Dentalium with special reference to the significance of the polar lobe V, VI. Differentiation of the cell pattern in lobeless embryos of Dentalium vulgare (da Costa) during larval development. Proc K Ned Akad Wet Ser C 79:245–266
van Dongen CAM, Geilenkirchen WLM (1975) The development of Dentalium with special reference to the significance of the polar lobe IV Division chronology and development of the cell pattern in Dentalium dentale after removal of the polar lobe at first cleavage. Proc K Ned Akad Wet Ser C 78:358–375
Dorresteijn AWC (1990) Quantitative analysis of cellular differentiation during early embryogenesis of Platynereis dumerilii. Roux's Arch Dev Biol 199:14–30
Dorresteijn AWC, Eich P (1991) Experimental change of cytoplasmic composition can convert determination of blastomeres in Platynereis dumerilii (Annelida, Polychaeta). Roux's Arch Dev Biol 200:342–351
Dorresteijn AWC, Fischer A (1988) The process of early development. Mikrofauna Mar 4:335–352
Fong PF, Kyozuka K, Abdelghani H, Hardege JD, Ram JL (1994) In vivo and in vitro induction of germinal vesicle breakdown in a freshwater bivalve, the zebra mussel Dreissena polymorpha (Pallas). J Exp Zool 269:467–474
Freeman G, Lundelius JW (1982) The developmental genetics of dextrality and sinistrality in the gastropod Lymnaea peregra. W Roux's Arch Entwicklungsmech Org 191:69–83
Freeman G, Lundelius JW (1992) Evolutionary implications of the mode of D quadrant specification in coelomates with spiral cleavage. J Evol Biol 5:205–247
Guerrier P (1970) Les caractères de la segmentation et la détermination de la polarité dorsoventrale dans le développement de quelques Spiralia III. Pholas dactylus et Spisula subtruncata (Mollusques Lamellibranches). J Embryol Exp Morphol 23:667–692
Henry JJ (1986) The role of unequal cleavage and the polar lobe in the segregation of developmental potential during the first cleavage in the embryo of Chaetopterus variopedatus. Roux's Arch Dev Biol 195:103–116
Kofoid CA (1894) On some laws of cleavage in Limax. Proc Am Acad Arts Sci 29:180–203
Lillie FR (1895) The embryology of the Unionidae. J Morphol 10: 1–76
Lillie FR (1901) The organization of the egg of Unio, based on a study of its maturation, fertilization and cleavage. J Morphol 17:227–293
Meisenheimer J (1901) Entwicklungsgeschichte von Dreissensia polyrnorpha Pall. Z Wiss Zool Abt A 4:1–161
Morgan TH, Tyler A (1930) The point of entrance of the spermatozoön in relation to the orientation to the embryo with spiral cleavage. Biol Bull 58:59–73
Orr-Weaver TL (1994) Developmental modification of the Drosophila cell cycle. Trends Genet 10:321–327
Pasteels J (1931) Recherche sur le déterminisme du mode de segmentation des mollusques lamellibranches (action des rayons ultraviolets sur l'oeuf de Barnea candida). Arch Biol 42: 389–413
Ram JL, Crawford GW Walker JV (1992) Zebra mussel spawning: release of eggs and sperm in response to external application of serotonin. Proceedings of the second international zebra mussel research conference, Rochester, New York, November 19–22, 1991. J Shellfish Res 11:236
Richardson KL, Jarret L, Finke EH (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35:313–323
Shimizu T (1993) Cleavage asynchrony in the Tubifex embryo: involvement of cytoplasmic and nucleus-associated factors. Dev Biol 157:191–204
Verdonk NH, van den Biggelaar JAM (1983) Early development and the formation of the germ layers. In: Verdonk NH, van den Biggelaar JAM, Tompa AS (eds) Mollusca, vol 3. Development. Academic Press, New York, pp 91–122
Verdonk NH, Cather JN (1983) Morphogenetic determination and differentiation. In: Verdonk NH, van den Biggelaar JAM, Tompa AS (eds) Mollusca, vol 3. Development. Academic Press, New York, pp 215–252
Wierzejski A (1905) Embryologic von Physa fontinalis L. Z Wiss Zool AN A 83:502–706
Wilson EB (1892) The cell-lineage of Nereis. A contribution to the cytogeny of the annelid body. J Morphol 6:361–480
Wilson EB (1904) Experimental studies on germinal localization. I. The germ regions in the egg of Dentalium. J Exp Zool 1:1–72
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Luetjens, C.M., Dorresteijn, A.W.C. Multiple, alternative cleavage patterns precede uniform larval morphology during normal development of Dreissena polymorpha (Mollusca, Lamellibranchia). Roux's Arch Dev Biol 205, 138–149 (1995). https://doi.org/10.1007/BF00357760
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00357760