Abstract
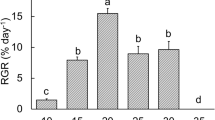
Six mediterranean macroalgae were cultivated for more than 2 yr under shade culture conditions, after which light requirements for growth were investigated at 16±2°C. The saturation light levels for growth in the logarithmic phase were related to the bathymetric distribution of the algae on the shore. The eulittoral to supralittoral red alga Bangia atropurpurea was saturated at a photon fluence rate of 71 μmol photons m-2 s-1, the upper sublittoral to eulittoral brown algae Scytosiphon lomentaria, Colpomenia peregrina and Kuckuckia spinosa and the sublittoral brown alga Stictyosiphon soriferus at 39 to 71 μmol photons m-2 s-1, and the deep-water alga Choristocarpus tenellus at 19 μmol photons m-2 s-1. The minimum light requirements for growth of B. atropurpurea and C. tenellus were determined by observing length increase for 56 d under limiting light conditions. The compensation and minimum irradiances required for growth of B. atropurpurea were 0.5 and 1 μmol photons m-2 s-1 respectively. The corresponding values for C. tenellus were 0.15 to 0.28 and 0.5 μmol photons m-2 s-1 respectively. C. tenellus was the siowest-growing species tested at saturating light conditions, but it grew faster than B. atropurpurea at 1 μmol photons m-2 s-1. Both B. atropurpurea and C. tenellus were able to survive 56 d in darkness, but only the latter grew under darkness in the first 14 d.
Similar content being viewed by others
Literature cited
Coppejans, E., Boudouresque, C.F. (1976). Présence de Choristocarpus tenellus (Kützing) Zanardini a Port-Cros. Trav. scient. Parc natn. Port-Cros 2: 195–197
Correa, J., Avilla, M., Santelices, B. (1985). Effects of some environmental factors on growth of sporelings in two species of Gelidium (Rhodophyta). Aquaculture 44: 221–227
Davison I.R. (1991). Environmental effects on algal photosynthesis: temperature. J. Phycol. 27: 2–8
Dring, M.J. (1981). Chromatic adaptation of photosynthesis in benthic marine algae: an examination of its ecological significance using a theoretical model. Limnol. Oceanogr. 26: 271–284
Falkowski, P. G., LaRoche, J. (1991). Acclimation to spectral irradiance in algae. J. Phycol. 27: 8–14
Fortes, M.D., Lüning, K. (1980). Growth rates of North sea macroalgae in relation to temperature, irradiance and photoperiod. Helgoländer Meeresunters. 34: 15–29
Geesink, R. (1973). Experimental investigations on marine and freshwater Bangia (Rhodophyta) from the Netherlands. J. exp. mar. Biol. Ecol. 11: 239–247
Geider, R.J., Osborne, B.A., Raven, J.A. (1985). Light dependence of growth and photosynthesis in Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 21: 609–619
Geider, R.J., Osborne, B.A., Raven, J.A. (1986). Growth, photosynthesis and maintenance metabolic coast in the diatom Phaeodactylum tricornutum at very low light levels. J. Phycol 22: 39–48
Guiry, M.D., Cunningham, E.M. (1984). Photoperiodic and temperature responses in the reproduction of north-eastern Atlantic Gigartina acicularis (Rhodophyta: Gigartinales). Phycologia 23: 357–367
Hanisak, M.D., Samuel, M.A., (1987). Growth rates in culture of several species of Sargassum from Florida, USA. Hydrobiologia 151/152: 399–404
Kain (Jones), J.M. (1969). The biology of Laminaria hyperborea. V. Comparison with early stages of competitors. J. mar. biol. Ass. U.K. 49: 455–473
Kain (Jones), J.M. (1987). Seasonal growth and photoinhibition in Plocamium cartilagineum (Rhodophyta) off the Isle of Man. Phycologia 26: 88–99
Kirk, J.T.O. (1983). Light and photosynthesis in aquatic ecosystems. Cambridge University Press, Cambridge
Larkum, A.W.D., Drew, E.A., Crosset, R.N. (1967). The vertical distribution of attached marine algae in Malta. J. Ecol. 55: 361–371
Levy, I., Gantt, E. (1988). Light acclimation in Porphyridium purpureum (Rhodophyta): growth, photosynthesis, and phycobilisomes. J. Phycol. 24: 452–458
Littler, M.M., Littler, D.S., Blair, S.M., Norris, J.N. (1985). Deepest known plant life discovered on an uncharted seamount. Science 227: 57–69
Lüning, K. (1981). Light. In: Lobban, C.S., Wynne, M.J. (eds.) The biology of seaweeds. Blackwell, Oxford, p. 326–355
Lüning, K. (1990). Seaweeds: their environment, biogeography and ecophysiology. John Wiley & Sons, New York
Maggs, C.A., Guiry, M.D. (1987). Environmental control of macroalgal phenology. In: Crawford, R.M.M. (ed.) Plant life in aquatic and amphibious habitats. Blackwell, Oxford, p. 359–373
Orfanidis, S. (1990). Temperature and photoperiodic responses of several Mediterranean macroalgae in relation to their distribution in the North Atlantic Ocean. Ph.D. thesis, University of Thessaloniki
Pérès, J.M., Molinier, R. (1957). Compte-rendu du colloque tenu à Gènes par le comité du Benthos de la Commission internationale pour L'Exploration scientifique de la mer Méditerranée. Recl. Trav. Stn mar. Endoume 13(22): 5–15
Ramus, J. (1981). The capture and transduction of light energy. In: Lobban, C.S., Wynne, M.J. (eds.) The biology of seaweeds. Blackwell, Oxford, p. 458–492
Ramus, J. (1983). A physiological test of the theory of complementary chromatic adaptation. II. Brown, green and red seaweeds. J. Phycol 19: 173–178
Ramus, J., van der Meer, J.P. (1983). A physiological test of the theory of complementary chromatic adaptation. I. Color mutant of a red seaweed. J. Phycol. 19: 86–91
Rosenvinge, L.K. (1935). On some Danish Phaeophyceae. Mém. Acad. R. Sci. Lett. Danm., Copenhagen (Sect. Sci.) 6: 1–40
Rueness, J., Tananger, T. (1984). Growth in culture of four red algae from Norway with potential for mariculture. Hydrobiologia 116/117: 303–307
Sand-Jensen, K. (1988). Minimum light requirements for growth in Ulva lactuca. Mar. Ecol. Prog. Ser. 50: 187–193
South, G.R., Hooper, R. (1976). Stictyosiphon soriferus (Phaeophyta, Dictyosiphonales) from eastern North Atlantic. J. Phycol 12: 24–29
Vermaat, J.E., Sand-Jensen, K. (1987). Survival, metabolism and growth and Ulva lactuca under winter conditions: a laboratory study of bottlenecks in the life cycle. Mar. Biol. 95: 55–61
Wiencke, C., tom Dieck, I. (1990). Temperature requirements for growth and survival of macroalgae from Antarctica and southern Chile. Mar. Ecol. Prog. Ser. 59: 157–170
Author information
Authors and Affiliations
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Orfanidis, S. Light requirements for growth of six shade-acclimated Mediterranean macroalgae. Marine Biology 112, 511–515 (1992). https://doi.org/10.1007/BF00356298
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00356298