Abstract
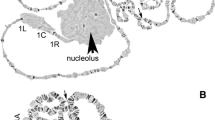
The chromosomal locations of four families of transposable elements, T1, Q, Pegasus and mariner, have been determined by in situ hybridization to polytene chromosomes of ovarian nurse cells of the mosquito Anopheles gambiae. As part of this effort, we have developed a vigorous pink-eyed laboratory strain of A. gambiae (PEST), rendered homozygous standard for chromosomal inversions on all autosomes. Ten different individuals of this strain were studied with each transposable element probe. The average number of hybridization sites per genome was 83.9 for T1, 63.4 for Q, 31.5 for Pegasus and 64.7 for mariner, excluding pericentric and centromeric regions. However, some degree of polymorphism was observed within each family such that, considering all ten individuals, 94 different sites were detected for T1, 82 sites for Q, 45 sites for Pegasus and 71 sites for mariner. The mean occupancy per site varied from 0.70 (Pegasus) to 0.91 (mariner), which, while significantly higher than that seen for transposable elements in natural populations of Drosophila melanogaster, is comparable to that seen in established laboratory stocks. In addition, these element families were not randomly distributed. All but Pegasus were concentrated in centromeric heterochromatin and centromere-proximal euchromatin, most showed a deficit of hybridization sites in the distal section of chromosomes, and a significant proportion of sites were coincident between families. These results provide the first detailed examination of the cytogenetic location of transposable elements in a nondrosophilid insect, and, through comparison with the behavior of transposable elements in Drosophila, may provide insight into the interaction between elements and host. The mapped elements are also expected to serve as landmarks useful in integrating the developing
Similar content being viewed by others
References
Benedict MQ, Seawright JA, Anthony DW, Avery SW (1979) Ebony, a semidominant lethal mutant in the mosquito Anopheles albimanus. Can J Genet Cytol 21:193–200
Benedict MQ, Besansky NJ, Chang H, Mukabayire O, Collins FH (1996) Mutations in the Anopheles gambiae pink-eye and white genes define distinct, tightly linked eye-color loci. J Heredity 87:48–53
Berg DE, Howe MM (1989) Mobile DNA. American Society for Microbiology, Washington, DC
Besansky NJ (1990a) A retrotransposable element from the mosquito Anopheles gambiae. Mol Cell Biol 10:863–871
Besansky NJ (1990b) Evolution of the T1 retroposon family in the Anopheles gambiae complex. Mol Biol Evol 7:229–246
Besansky NJ, Powell JR (1992) Reassociation kinetics of Anopheles gambiae (Diptera: Culicidae) DNA. J Med Entomol 29: 125–128
Besansky NJ, Paskewitz SM, Mills-Hamm D, Collins FH (1992) Distinct families of site-specific retrotransposons occupy identical positions in the rRNA genes of Anopheles gambiae. Mol Cell Biol 12:5102–5110
Besansky NJ, Bedell JA, Mukabayire O (1994) Q: a new retrotransposon from the mosquito Anopheles gambiae. Insect Mol Biol 3:49–56
Besansky NJ, Bedell JA, Benedict MQ, Mukabayire O, Hilfiker D, Collins FH (1995) Cloning and characterization of the white gene from Anopheles gambiae. Insect Mol Biol 4:217–231
Besansky NJ, Mukabayire O, Bedell J, Lusz H (1996) Pegasus, a small terminal inverted repeat transposable element found in the white gene of Anopheles gambiae. Genetica (in press)
Biémont C (1986) Polymorphism of the Mdg-1 and I mobile elements in Drosophila melanogaster. Chromosoma 93:393–397
Biémont C (1992) Population genetics of transposable DNA elements: a Drosophila point of view. Genetica 86:67–84
Biémont C, Lemeunier F, Garcia Guerreiro MP, Brookfield JF, Gautier C, Aulard S, Pasyukova EG (1994) Population dynamics of the copia, mdg1, mdg3, gypsy, and P transposable elements in a natural population of Drosophila melanogaster. Genet Res 63:197–212
Blackman RK, Gelbart WM (1989) The transposable element hobo of Drosophila melanogaster. In: Berg DE, Howe MM (eds) Mobile DNA. American Society for Microbiology, Washington, DC, pp 523–529
Blackman RK, Koehler MMD, Grimalia R, Gelbart WM (1989) Identification of a fully-functional hobo transposable element and its use for germ-line transformation of Drosophila. EMBO J 8:211–217
Blinov AG, Sobanov YV, Gaidamakova EK, Bogachev SS, Kolesnikov NN, Filippova MA, Kiknadze II (1991) MEC: a transposable element from Chironomus thummi (Diptera). Mol Gen Genet 229:152–154
Brookfield JFY (1995) Transposable elements as selfish DNA. In: Sherratt DJ (ed) Mobile genetic elements. IRL Press, Oxford New York Tokyo, pp 130–153
Charlesworth B, Lapid A (1989) A study of ten families of transposable elements on X chromosomes from a population of Drosophila melanogaster. Genet Res 54:113–125
Collins FH, Besansky NJ (1994) Vecor biology and the control of malaria in Africa. Science 264:1874–1875
Coluzzi M, Sabatini A, Petrarca V, Di Deco MA (1979) Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg 73:483–497
Curtis CF (1994) The case for malaria control by genetic manipulation of its vectors. Parasitol Today 10:371–374
Deininger PL, Batzer MA, Hutchison CA, Edgell MH (1992) Master genes in mammalian repetitive DNA amplification. Trends Genet 8:307–311
Engels WR (1989) P elements in Drosophila melanogaster. In: Berg DE, Howe MM (eds) Mobile DNA. Am soc Microbiol, Washington, DC, pp 437–484
Finnegan DJ (1989) The I factor and I-R hybrid dysgenesis in Drosophila melanogaster. In: Berg DE, Howe MM (eds) Mobile DNA. Am Soc Microbiol, Washington, DC, pp 503–517
Githeko AK, Brandling-Bennett AD, Beier M, Atieli F, Owaga M, Collins FH (1992) The reservoir of Plasmodium falciparum malaria in a holoendemic area of western Kenya. Trans R Soc Trop Med Hyg 86:355–358
Green MM (1988) Mobile DNA elements and spontaneous gene mutation. In: Lambert ME, McDonald JF, Weinstein IB (eds) Banbury Report 30: Eukaryotic transposable elements as mutagenic agents. Cold Spring Harbor, Laboratory Press, Cold Spring Harbor, NY, pp 41–50
Hankeln Th, Schmidt ER (1990) New foldback transposable element TFB1 found in histone genes of the midge Chironomus thummi J Mol Biol 215:477–482
Hey J (1989) The transposable portion of the genome of Drosophila algonquin is very different from that in Drosophila melanogaster. Mol Biol Evol 6:66–79
Holliday R (1982) Gene conversion: a possible mechanism for eliminating selfish DNA. In: Lemont JF, Generoso WM (eds) Molecular and cellular mechanisms of mutagenesis. Plenum, New York, pp 259–264
Kidwell MG (1992) Horizontal transfer. Curr Opin Genet Dev 2:868–873
Kidwell MG, Ribeiro JMC (1992) Can transposable elements be used to drive disease refractoriness genes into vector populations? Parasitol Today 8:325–329
Kozlova TY, Semeshin VF, Tretyakova IV, Kokoza EB, Pirrotta V, Grafodatskaya VE, Belyaeva ES, Zhimulev IF (1994) Molecular and cytogenetical characterization of the 10A1-2 band and adjoining region in the Drosophila melanogaster polytene X chrosome. Genetics 136: 1063–1073
Kumar V, Collins FH (1994) A technique for nucleic acid in situ hybridization to polytene chromosomes of mosquitoes in the Anopheles gambiae complex. Insect Mol Biol 3:41–47
Langley CH, Montgomery EA, Hudson R, Kaplan NL, Charlesworth B (1988) On the role of unequal exchange in the containment of transposable element copy number. Genet Res 52:223–236
Leigh-Brown AJ, Moss JE (1987) Transposition of the I element and copia in a natural population of Drosophila melanogaster. Genet Res 49:121–128
Lidholm D-A, Lohe AR, Hartl DL (1993) The transposable element mariner mediates germline transformation in Drosophil melanogaster. Genetics 134:859–868
Louis C, Yannopoulos G (1988) The transposable elements involved in hybrid dysgenesis in Drosophila melanogaster. Oxf Surv Eukaryotic Genes 5:205–250
Loukeris TG, Livadaras I, Arca B, Savakis C (1995) The white gene of Ceratitis capitata: a phenotypic marker for germline transformation. Science 270:2005–2008
Lyttle TW, Haymer DS (1992) The role of the transposble element hobo the origin of endemic inversions in wild populations of Drosophila melanogaster. Genetica 86:113–126
Maruyama K, Hartl DL (1991) Evolution of the transposable element mariner in Drosophila species. Genetics 128:319–329
Mason JM, Biessmann H (1995) The unusual telomeres of Drosophila. Trends Genet 11:58–62
Montgomery EA, Langley CH (1983) Transposable elements in Mendelian populations. II. Distribution of three copia-like elements in natural population of Drosophila melanogaster. Genetics 104:473–483
Robertson HM (1993) The mariner transposable element is widespread in insects. Nature 362:241–245
Robertson HM, Lampe DJ (1995) Distribution of transposable elements in arthropods. Annu Rev Entomol 40:333–357
Rubin GM, Spradling AC (1982) Genetic transformation of Drosophila with transposable element vectors. Science 218:348–353
Rudkin GT (1972) Replication in polytene chromosomes. Results Probl Cell Differ 4:59–85
Schmidt ER (1984) Clustered and interspersed repetitive DNA sequence family of Chironomus. J Mol Biol 178:1–15
Simmons GM (1992) Horizontal tranfer of hobo transposable elements within the Drosophila melanogaster species complex: evidence from DNA sequencing. Mol Biol Evol 9:1050–1060
Spierer PA (1984) A molecular approach to chromosome organization. Dev Genet 4:333–339
Spradling AC, Rubin GM (1982) Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218: 341–347
Strand, DJ, McDonald JF (1989) Insertion af a, copia element 5′ to the D. melanogaster alcohol dehydrogenase gene (adh) is associated with altered developmental and tissue-specific patterns of expression. Genetics 121:787–794
Strobel E, Dunsmuir P, Rubin GM (1979) Polymorphisms in the chromosomal locations of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell 17:429–439
Weiner AM, Deininger PL, Efstratiadis A (1986) Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem 55:631–661
Wobus U, Baumlein H, Bogachev SS, Borisevich IV, Panitz R, Kolesnikov NN (1990) A new transposable element in Chironomus thummi. Mol Gen Genet 222:311–316
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukabayire, O., Besansky, N.J. Distribution of T1, Q, Pegasus and mariner transposable elements on the polytene chromosomes of PEST, a standard strain of Anopheles gambiae . Chromosoma 104, 585–595 (1996). https://doi.org/10.1007/BF00352298
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00352298