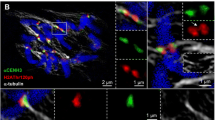
Abstract
Recognition of homologous chromosomes during meiotic prophase is associated in most cases with the formation of the synaptonemal complex along the length of the chromosome. Telomeres, located at the nuclear periphery, are preferential initiation sites for the assembly of the synaptonemal complex. In most eukaryotic cells, telomeres cluster in a restricted area, leading to the “bouquet” configuration in leptotene-zygotene, while this typical organization progressively disappears in late zygotene-pachytene. We wondered whether such striking changes in the intranuclear ordering and pairing of meiotic chromosomes during the progression of prophase I could be correlated with activity of the centrosome and/or microtubule-organizing center (MTOC). Plant cells may be used as a model of special interest for this study as the whole nuclear surface acts as an MTOC, unlike other cell types where MTOCs are restricted to centrosomes or spindle pole bodies. Using a monoclonal antibody (mAb 6C6) raised against isolated calf centrosomes we found that the 6C6 antigen is present over the entire surface of the plant meiotic nucleus, in early prophase I, before chromosomal pairing. At zygotene, short fragments of chromosomes become stained near the nuclear envelope and within the nucleus. At pachytene, after complete synapsis, the labeling specifically concentrates within the synaptonemal complexes, although the nuclear surface is no longer reactive. Ultrastructural localization using immunogold labeling indicates that the 6C6 antigen is colocalized with the synaptonemal complex structures. Later in metaphase I, the antigen is found at the kinetochores. Our data favor the idea that the 6C6 antigen may function as a particular “chromosomal passenger-like’ protein. These observations shed new light on the molecular organization of the plant synaptonemal complex and on the redistribution of cytoskeleton-related antigens during initiation of meiosis. They suggest that antigens of MTOCs are relocated to chromosomes during the synapsis process starting at telomeres and contribute to the spatial arrangement of meiotic chromosomes. Such cytoskeleton-related antigens may acquire different functions depending on their localization, which is cell-cycle regulated.
Similar content being viewed by others
References
Abirached-Darmency M, Zickler D, Cauderon Y (1983) Synaptonemal complex and recombination nodules in rye (Secale cereale). Chromosoma 88:299–306
Bajer AS, Mole-Bajer J (1986) Reorganization of microtubules in endosperm cells and cell fragments of the higher plant Haemanthus in vivo. J Cell Biol 102:263–281
Cao L, Alani E, Kleckner N (1990) A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell 61:1089–1101
Carpenter ATC (1987) Gene conversion, recombination nodules, and the initiation of meiotic synapsis. Bioessays 6:232–236
Chen Q, Pearlman RE, Moens P (1992) Isolation and characterization of a cDNA encoding a synaptonemal complex protein. Biochem Cell Biol 70:1030–1038
Chevrier V, Komesli L, Schmit AC, Vantard M, Lambert AM, Job D (1992) A monoclonal antibody, raised against mammalian centrosomes and screened by recognition of plant microtubule-organizing centers, identifies a pericentriolar component in different cell types. J Cell Sci 101:823–835
Chikashige Y, Ding DQ, Funabiki H, Haraquchi T, Mashika S, Yanagida M, Hiraoka Y (1994) Telomere-led premeiotic chromosome movement in fission yeast. Science 264:270–273
Compton DA, Szilak J, Cleveland DW (1992) Primary structure of NuMA, an intranuclear protein that defines a novel pathway for segregation of proteins at mitosis. J Cell Biol 116:1395–1408
De Jong JH, van Eden J, Sybenga J (1989) Synaptonemal complex formation and metaphase I configuration patterns in a translocation heterozygote of rye (Secale cereale). Genome 32:72–81
De Mey J, Lambert AM, Bajer AS, Moeremans M, De Brabander M (1982) Visualization of microtubules in interphase and mitotic plant cells of Haemanthus endosperm with the immunogold staining method. Proc Natl Acad Sci USA 79:1898–1902
Dobson MJ, Pearlman RE, Karaiskakis A, Spyropoulos B, Moens PB (1994) Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J Cell Sci 107: 2749–2760
Dresser ME (1987) Synaptonemal complex and meiosis: an immunocytochemical approach. In: Moens PB (ed) Meiosis. Academic Press, New York, pp 245–274
Earnshaw WC, Bernat RL (1991) Chromosomal passengers: Toward an integrated view of mitosis. Chromosoma 100:139–146
Engelbrecht J, Roeder GS (1990) MER1, a yeast gene required for chromosome pairing and genetic recombination. Genetics 121:237–247
Fussel CP (1987) The Rabl orientation: a prelude to synapsis. In: Moens PB (ed) Meiosis. Academic Press, New York, pp 275–299
Gil-Alberti L, del Mazo J (1992) Microtubule-associated proteins during mouse spermatogenesis: localization of a protein immunologically related to brain MAP1B protein in the synaptonemal complex. Cytogenet Cell Genet 59:1–5
Gillies CB (1984) The synaptonemal complex in higher plants. CRC Crit Rev Plant Sci 2:81–116
Gilson E, Laroche T, Gasser SM (1993) Telomeres and the functional architecture of the nucleus. Trends Cell Biol 3:128–134
Herickhoff L, Stack S, Sherman J (1993) The relationship between synapsis, recombination nodules and chiasmata in tomato translocation heterozygotes. Heredity 71:373–385
Heyting C, Dietrich AJJ, Redeker EJW, Vink ACG (1985) Structure and composition of synaptonemal complexes, isolated from rat spermatocytes. Eur J Cell Biol 36:307–314
Heyting C, Moens PB, van Raamsdonk W, Dietrich AJJ, Vink ACG, Redeker EJW (1987) Identification of two major components of the lateral elements of synaptonemal complexes of the rat. Eur J Cell Biol 43:148–154
Heyting C, Dietrich AJJ, Moens PB, Dettmers J, Offenberg H, Redeker EJW, Vink ACG (1988) Synaptonemal complex proteins. Genome 31:81–87
Hotta Y, Shepard J (1973) Biochemical aspects of colchicine action on meiotic cells. Mol Gen Genet 122:243–260
John B (1990) Meiosis. Cambridge University Press, Cambridge
Kohli J, Bähler J (1994) Homologous recombination in fission yeast: absence of crossover interference and synaptonemal complex. Experientia 50:295–306
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680–685
Lambert AM (1993) Microtubule-organizing centers in higher plants. Curr Opin Cell Biol 5:116–122
Lambert AM, Bajer AS (1977) Microtubule distribution and reversible arrest of chromosome movement induced by low temperature. Cytobiologie 15:1–23
Loidl J (1990) The initiation of meiotic chromosome pairing: the cytological view. Genome 3:759–778
Loidl J (1994) Cytological aspects of meiotic recombination. Experientia 5:285–294
Mizuno K (1993) Microtubule nucleation sites on nuclei of higher plant cells. Protoplasma 173:77–85
Moens PB (1969) The fine structure of meiotic chromosome polarization and pairing in Locusta migratoria spermatocytes. Chromosoma 2:1–25
Moens PB, Earnshaw WC (1989) Anti-topoisomease II recognizes meiotic chromosome cores. Chromosoma 98:317–322
Moens PB, Heyting C, Dietrich AJJ, van Raamsdonk W, Chen Q (1987) Synaptonemal complex antigen localization and conservation. J Cell Biol 105:93–103
Moens PB, Spyropoulos B, Dobson MJ, Karaiskakis A, Pearlman RE (1992) Searching for synaptonemal complex proteins and their genes. Dev Genet 13:435–439
Moses MJ (1968) Synaptinemal complex. Annu Rev Genet 2:363–412
Offenberg HH, Dietrich AJJ, Heyting C (1991) Tissue distribution of two major components of the synaptonemal complexes of rat. Chromosoma 101:83–91
Ohyama T, Iwaikawa Y, Kobayashi T, Hotta Y, Tabata S (1992) Isolation of synaptonemal complexes from lily microsporocytes. Plant Sci 86: 115–124
Padmore R, Cao L, Kleckner N (1991) Temporal comparison of recombination and synaptonemal complex formation during meiosis in S. cerevisiae. Cell 66:1239–1256
Petes TD, Malone RE, Symington LS (1991) Recombination in yeast. In: Broach JR, Pringle JR, Johnes EW (eds) The molecular and cellular biology of the yeast Saccharomyces, vol 1. Cold spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 407–521
Rasmussen SW, Holm PB (1980) Mechanics of meiosis. Hereditas 93:187–216
Roeder GS (1990) Chromosome synapsis and genetic recombination: their roles in meiotic chromosome segregation. Trends Genet 6:385–389
Salonen K, Paranko J, Parvinen M (1982) A Colcemid-sensitive mechanism involved in regulation of chromosome movements during meiotic pairing. Chromosoma 85:611–618
Scherthan H, Bähler J, Kohli J (1994) Dynamics of chromosome organization and pairing during meiotic prophase in fission yeast. J Cell Biol 127: 273–285
Schmit AC, Stoppin V, Chevrier V, Job D, Lambert AM (1994) Cell cycle dependent distribution of a centrosomal antigen at the perinuclear MTOC or at the kinetochores of higher plant cells. Chromosoma 103:343–351
Smith A, Benavente R (1992) Identification of a structural protein component of rat synaptonemal complex. Exp Cell Res 198:291–297
Stoppin V, Vantard M, Schmit AC, Lambert AM (1994) Isolated maize nuclei can nucleate microtubule assembly: the nuclear surface in higher plants has a centrosome-like activity. Plant Cell 6:1099–1106
Sym M, Roeder GS (1995) ZIP 1-induced changes in synaptonemal complex structure and polycomplex assembly. J Cell Biol 128:455–466
Sym M, Engelbrecht J, Roeder GS (1993) ZIP1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell 72:365–378
Vantard M, Peter C, Fellous A, Schellenbaum P, Lambert AM (1994) Characterization of a 100 kDa heat-stable microtubule-associated protein from higher plants. Eur J Biochem 220: 847–853
von Wettstein D, Rasmussen SW, Holm PB (1984) The synaptonemal complex in genetic segregation. Annu Rev Genet 18:331–413
Weiner BM, Kleckner N (1994) Chromosome pairing via multiple interstitial interactions before and during meiosis in yeast. Cell 77:977–991
Westergard M, von Wettstein D (1972) The synaptonemal complex. Annu Rev Genet 6:71–110
Zickler D (1977) Development of the synaptonemal complex and the recombination nodules during meiotic prophase in the seven bivalents of the fungus Sordaria macrospora Auersw. Chromosoma 61:289–316
Zickler D, Moreau P, Huynh AD, Slezec A (1992) Correlation between pairing initiation sites, recombination nodules and meiotic recombination in Sordaria macrospora. Genetics 132: 135–148
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmit, AC., Endlé, MC. & Lambert, AM. The perinuclear microtubule-organizing center and the synaptonemal complex of higher plants share a common antigen: its putative transfer and role in meiotic chromosomal ordering. Chromosoma 104, 405–413 (1996). https://doi.org/10.1007/BF00352264
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00352264