Abstract
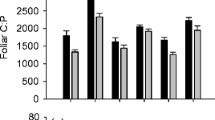
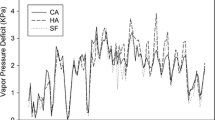
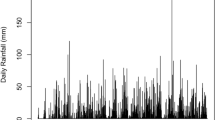
Water and salinity relations were evaluated in recovering burned individuals of the dominant woody taxa from low-elevation riparian plant communities of the southwestern U.S. Soil elemental analyses indicated that concentrations of most nutrients increased following fire, contributing to a potential nutrient abundance but also elevated alluvium salinity. Boron, to which naturalized Tamarix ramosissima is tolerant, was also elevated in soils following fire. Lower moisture in the upper 30 cm of burned site soil profiles was attributed to shifts in evapotranspiration following fire. Higher leaf stomatal conductance occurred in all taxa on burned sites. This is apparently due to higher photosynthetic photon flux density at the midcanopy level and may be partially mitigated by reduced unit growth in resprouting burned individuals. Predawn water potentials varied little among sites, as was expected for plants exhibiting largely phreatophytic water uptake. Midday water potentials in recovering Salix gooddingii growing in the Colorado River floodplain reached levels which are considered stressful. Decreased hydraulic efficiency was also indicated for this species by examining transpiration-water potential regressions. Recovering, burned Tamarix and Tessaria sericea had enriched leaf tissue δ13C relative to unburned controls. Higher water use efficiency following fire in these taxa may be attributed to halophytic adaptations, and to elevated foliar nitrogen in Tessaria. Consequently, mechanisms are proposed which would facilitate increased community dominance of Tamarix and Tessaria in association with fire. The theory that whole ecosystem processes are altered by invading species may thus be extended to include those processes related to disturbance.
Similar content being viewed by others
References
Al-Hilli MR (1987) Remote sensing and the ecology of vegetational changes in the Fohood area. Iraq J Agric Water Resour Res Plant Prod 6:17–38
Anderson JE (1982) Factors controlling transpiration and photosynthesis in Tamarix chinensis Lour. Ecology 63:48–56
Bahre CJ (1985) Wildfire in southeastern Arizona between 1859 and 1890. Desert Plants 7:190–194
Berry WL (1970) Characteristics of salts secreted by Tamarix aphylla. Am J Bot 57:1226–1230
Busch DE, Ingraham NL, Smith SD (1992) Water relations in woody riparian phreatophytes of the southwestern U.S.: a stable isotope study. Ecol Applic 2:450–459
Chapin FS (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Chapman HD, Pratt PF (1962) Methods of analysis for soils, plants, and waters. Univ Calif Press, Berkeley, 309 pp
Conover WJ (1980) Practical Nonparametric Statistics. John Wiley and Sons, New York, 493 pp
Crins WJ (1989) The Tamaricaceae in the southeastern United States. J Arnold Arbor 70:403–425
Dinerstein E (1979) An ecological survey of the Royal Karnali-Bardia Wildlife Reserve, Nepal, Part I: Vegetation, modifying factors, and successional relationships. Biol Conserv 15:127–150
Dionigi CP, Mendelssohn IA, Sullivan VI (1985) Effects of soil waterlogging on the energy status and distribution of Salix nigra and S. exiqua (Salicaceae) in the Atchafalaya River Basin of Louisiana. Am J Bot 72:109–119
Dobyns HF (1981) From fire to flood: historic human destruction of Sonoran Desert riverine oases. Ballena Press, Socorro NM, 222 pp
Ehleringer JR, Cooper TA (1988) Correlations between carbon isotope ratio and microhabitat in desert plants. Oecologia 76:562–566
Ehleringer JR, Phillips SL, Comstock JP (1992) Seasonal variation in the carbon isotopic composition of desert plants. Func Ecol 6:396–404
Elfving DC, Kaufmann MR, Hall AE (1972) Interpreting leaf water potential measurements with a model of the soil-plant-atmosphere continuum. Physiol Plant 27:161–168
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Farquhar GD, Ball MC, von Caemmerer S, Roksandic Z (1982) Effect of salinity and humidity on δ13C value of halophytes-evidence for diffusional isotope fractionation determined by the ratio of intercellular/atmospheric partial pressure of CO2 under different environmental conditions. Oecologia 52:121–124
Fowells HA (1965) Silvics of forest trees of the United States. Agric Handbook No 271. USDA For Serv, Washington DC, 762 pp
Gary HL (1963) Root distribution of five-stamen tamarisk, seepwillow, and arrowweed. For Sci 9:311–314
Hodgkinson KC (1992) Water relations and growth of shrubs before and after fire in a semiarid woodland. Oecologia 90:467–473
Jackson J, Ball JT, Rose MR (1990) Assessment of the salinity tolerance of eight Sonoran Desert riparian trees and shrubs. US Bureau of Reclamation, Yuma, AZ, 102 pp
Kelliher FM, Kirkham MB, Tauer CG (1980) Stomatal resistance, transpiration, and growth of drought-stressed eastern cotton-wood. Can J For Res 10:447–451
Kirby RE, Lewis SJ, Sexson TN (1988) Fire in North American wetland ecosystems and fire-wildlife relations: An annotated bibliography. Biol Rep 88(1), US Fish and Wildl Serv, Washington, DC, 146 pp
Le Houerou HN (1981) Impact of man and his animals on Meditteranean vegetation. In: di Castri F, Goodall DW, Specht RL (eds) Ecosystems of the world 11 (Mediterranean-type shrublands). Elsevier, Amsterdam Oxford New York, pp 479–521
McQueen IS, Miller RF (1972) Soil-moisture and Energy Relationships Associated with Riparian Vegetation near San Carlos, Arizona. Prof Paper 655-E, US Geol Surv, Washington, DC, 51 pp
Montgomery DC (1984) Design and analysis of experiments. Wiley, New York Chichester Brisbane Toronto Singapore, 538 pp
Moser EB, Saxton AM, Pezeshki SR (1990) Repeated measures analysis of variance: application to tree resarch. Can J For Res 20:524–535
Mutch RW (1970) Wildland fires and ecosystems: an hypothesis. Ecology 51:1046–1051
Neter J, Wasserman W, Kutner MM (1989) Applied Linear Regression Models. Richard D. Irwin, Homewood, IL, 667 pp
Ohmart RD, Anderson BW (1982) North American desert riparian ecosystems. In: Bender GL (ed) Reference handbook on the deserts of North America. Greenwood Press, Westport, CN, pp 433–479
Pallardy SG, Kozlowski TT (1981) Water relations of Populus clones. Ecology 62:159–169
Potvin C, Lechowicz MJ, Tardif S (1990) The statistical analysis of ecophysiological response curves obtained from experiments involving repeated measures. Ecology 71:1389–1400
Reich PB, Hinckley TM (1989) Influence of pre-dawn water potential and soil-to-leaf hydraulic conductance on maximum daily leaf diffusive conductance in two oak species. Func Ecol 3:719–726
Reich PB, Abrams MD, Ellsworth DS, Kruger EL, Tabone TJ (1990) Fire affects ecophysiology and community dynamics of central Wisconsin oak forest regeneration. Ecology 71:2179–2190
Rundel PW (1981) Fire as an ecological factor. In: Lange OL, Nobel PS, Osmond CB Ziegler H (eds) Physiological plant ecology I. (Encyclopedia of Plant Physiology, NS, vol 12 B). Springer, Berlin Heidelberg New York, pp 501–538
Schulte PJ, Hinckley TM, Stettler RF (1987) Stomatal responses of Populus to leaf water potential. Can J Bot 65:255–260
Schulze E-D, Hall AE (1982) Stomatal responses, water loss and CO2 assimilation rates of plants in contrasting environments. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology II. (Encyclopedia of plant physiology, NS, vol 12 B) Springer, Berlin Heidelberg New York, pp 181–230
Schulze E-D, Cermak J, Matyssek R, Penka M, Zimmermann R, Vasicek F, Gries W, Kucera J (1985) Canopy transpiration and water fluxes in the xylem of the trunk of Larix and Picea trees — a comparison of xylem flow, porometer and cuvette measurements. Oecologia 66:475–483
Smith BN, Oliver J, McMillan C (1976) Influence of carbon source, oxygen concentration, light intensity, and temperature on 13C/12C ratios in plant tissues. Bot Gaz 137:99–104
Smith SD, Nobel PS (1986) Deserts. In: Baker NR, Long SP (eds) Photosynthesis in contrasting environments (Topics in Photosynthesis, vol 7). Elsevier, Amsterdam Oxford New York, pp 13–62
Swetnam TW (1990) Fire history and climate in the southwestern United States. In: Krammes JS (ed) Effects of fire management on Southwestern natural resources. USDA For Serv, Gen Tech Rep RM-191. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO, pp 6–17
Szaro RC (1989) Riparian forest and scrubland types of Arizona and New Mexico. Desert Plants 9:70–138
Topp GC, Davis JL (1985) Measurement of soil water content using time-domain reflectometry (TDR): a field evaluation. Soil Sci Soc Am J 49:19–24
Turner NC (1988) Measurement of plant water status by the pressure chamber technique. Irrig Sci 9:289–308
U.S. Salinity Laboratory Staff (1954) Diagnosis and improvement of saline and alkaline soils. USDA Handbook 60. U.S. Government Printing Office, Washington, DC, 160 pp
Vitousek PM (1986) Biological invasions and ecosystem properties: Can species make a difference? In: Mooney HA and Drake JA (eds) Ecology of biological invasions of North America and Hawaii. Springer, Berlin Heidelberg New York, pp 163–176
Vitousek PM (1990) Biological invasions and ecosystem processes: towards an integration of population biology and ecosystem studies. Oikos 57:7–13
Wellington AB (1984) Leaf water potentials, fire and the regeneration of mallee eucalypts in semi-arid, south-eastern Australia. Oecologia 64:360–362
Whisenant SG, Scifres CJ, Ueckert DN (1984) Soil water and temperature response to prescribed burning. Great Basin Nat 44:558–562
Wright HA, Bailey AW (1982) Fire ecology, United States and southern Canada. Wiley, New York Chichester Brisbane Toronto Singapore, 501 pp
Zar JH (1983) Biostatistical analysis. Prentice-Hall, Englewood Cliffs, NJ, 718 pp
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Busch, D.E., Smith, S.D. Effects of fire on water and salinity relations of riparian woody taxa. Oecologia 94, 186–194 (1993). https://doi.org/10.1007/BF00341316
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00341316