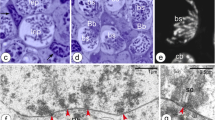
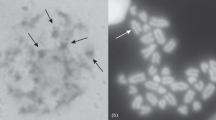
Abstract
The identification and progression of the prophase stages of meiosis in the mouse foetal ovary are reported, from d 13 of gestation to d 1 postpartum. Air-dried Giemsa-stained oocyte preparations are compared with surface-spread silver-stained cells. The latter method allows a more detailed quantitative analysis of the pachytene stage. Numbers of synaptonemal complexes can be counted, and the degree of synapsis determined. The progression of cells appears to be relatively synchronous, in agreement with previous reports. The activity of nucleolar organisers, in particular one associated with the shortest synaptonemal complex (chromosome No. 19) is described. At late pachytene the lateral elements of the No. 19 bivalent desynapse precociously with apparent nucleolar involvement.
Similar content being viewed by others
References
Baker, T.G.: Oogenesis and ovulation. In “Reproduction in mammals.” I. Germ cells and fertilization. (C.R. Austin, R.V. Short, eds.) Cambridge University Press (1972)
Bakken, A.H., McClanahan, M.: Patterns of RNA synthesis in early meiotic prophase oocytes from fetal mouse ovaries. Chromosoma (Berl.) 67, 21–40 (1978)
Borum, K.: Oogenesis in the mouse. A study of the meiotic prophase. Exp. Cell Res. 24, 495–507 (1961)
Borum, K.: Oogenesis in the mouse. Exp. Cell Res. 45, 39–47 (1966)
Crone, M., Levy, E., Peters, H.: The duration of the premeiotic DNA synthesis in mouse oocytes. Exp. Cell Res. 39, 678–688 (1965)
Darlington, C.D.: Recent advances in cytology. London. Churchill (1965)
Dresser, M.E., Moses, M.J.: Silver staining of synaptonemal complexes in surface spreads for light and electron microscopy. Exp. Cell Res. 121, 416–419 (1979)
Elsevier, S.M., Ruddle, F.H.: Localisation of genes coding for 18 S and 28 S ribosomal RNA within the genome of Mus musculus. Chromosoma (Berl.) 52, 219–228 (1975)
Fabricant, J.D., Schneider, E.L.: Studies of the genetic and immunologic components of the maternal age effect. Dev. Biol. 66, 337–343 (1978)
Fletcher, J.M.: Light microscope analysis of meiotic prophase chromosomes by silver staining. Chromosoma (Berl.) 72, 241–248 (1979)
Gosden, R.G.: Chromosomal anomalies of preimplantation mouse embryos in relation to maternal age. J. Reprod. Fert. 35, 351–354 (1973)
Henderson, S.A., Edwards, R.G.: Chiasma frequency and maternal age in mammals. Nature (Lond.) 218, 22–28 (1968)
Holm, P.B., Rasmussen, S.W., Wettstein, D. von: The possible contribution of electron microscopy to the understanding of the mechanism of non-disjunction in man. Mut. Res. 61, 115–119 (1979)
Howell, W.M., Black, D.A.: Controlled silver-staining of nucleolus organizer-regions with a protective colloidal developer: a 1-step method. Experientia (Basel) 36, 1014–1015 (1980)
Jagiello, G., Fang, J.S.: Analysis of diplotene chiasma frequencies in mouse oocytes and spermatocytes in relationship to ageing and sexual dimorphism. Cytogenet. Cell Genet. 23, 53–60 (1979)
Jones, G.H., Wallace, B.M.N.: Meiotic chromosome pairing in Stethophyma grossum spermatocytes studied by a surface-spreading and silver-staining technique. Chromosoma (Berl.) 78, 187–201 (1980)
Mirre, C., Hartung, M., Stahl, A.: Association of ribosomal genes in the fibrillar center of the nucleolus: A factor influencing translocation and non disjunction in the human meiotic oocyte. Proc. natl. Acad. Sci. USA, 77, 6017–6021 (1980)
Moses, M.J.: Microspreading and the synaptonemal complex in cytogenetic studies. Chromosomes today 6, 71–82 (1977)
Moses, M.J., Slatton, G.H., Gambling, T.M., Starmer, C.F.: Synaptonemal complex karyotyping in spermatocytes of the Chinese hamster (Cricetulus griseus). III. Quantitative evaluation. Chromosoma (Berl.) 60, 345–375 (1977)
Peters, H., Levy, E., Crone, M.: Deoxyribonucleic acid synthesis in oocytes of mouse embryos. Nature 195, 915–916 (1962)
Solari, A.J.: Synaptonemal complexes and associated structures in microspread human spermato cytes. Chromosoma (Berl.) 81, 315–337 (1980)
Solari, A.J., Counce, S.J.: Synaptonemal complex karyotyping in Melanoplus differentialis. J. Cell Sci. 26, 229–250 (1977)
Stahl, A., Luciani, J.M.: Nucleoli and chromosomes: Their relationship during the meiotic prophase of the human fetal oocyte. Humangenetik 14, 269–284 (1972)
Tres, L.L.: Extensive pairing of the XY bivalent in mouse spermatocytes as visualized by whole-mount electron microscopy. J. Cell Sci., 25, 1–15 (1977)
Winking, H., Nielsén, K., Gropp, A.: Variable positions of NORs in Mus musculus. Cytogenet. Cell Genet. 26, 158–164 (1980)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Speed, R.M. Meiosis in the foetal mouse ovary. Chromosoma 85, 427–437 (1982). https://doi.org/10.1007/BF00330366
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00330366