Abstract
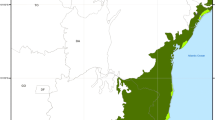
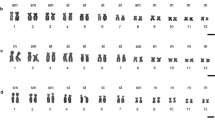
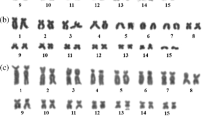
Atractomorpha similis (2 n=19 ♂, 20 ♀) is a hygrophilous, tropical to temperate, species of pyrgomorphine grasshopper. We have sampled 70 populations covering the known distributional range of this species within Australia. All of them proved to be polymorphic for heterochromatin content as revealed by C-band analysis of embryonic neuroblasts. This polymorphism affects all ten members of the basic haploid set and includes variants involving differences in either the presence or the amount of procentric, interstitial and terminal C-blocks, as well as variation in the occurrence and nature of short arms on otherwise telocentric chromosomes. A majority of these variants appear to result from heterochromatin addition since the presumptive sibling, Atractomorpha australis, like other species of the genus that have been C-banded, is generally depauperate in heterochromatin. The net result of this extraordinary polymorphism is that each chromosome of A. similis exists in 10–50 distinct morphs. Consequently, there is a high level of chromosomal heterozygosity in all populations in terms of the number of heterozygous pairs present within a complement and an even higher level in terms of the total range of karyomorph patterns. There is also a wide range of total heterochromatin content, as measured by the percent of the total chromosome area occupied by C-band material, with values ranging from 13% to 44%. Specific marker chromosomes which predominate in particular geographical areas serve to distinguish six major cytotypes within A. similis. The two most southerly of these cytotypes show a narrower range of heterochromatin content but with higher values which reflect the more general occurrence of substantial terminal C-blocks within them. Finally, the populations from Fraser Island constitute a particularly distinctive cytotype characterised by the least number of morphs, the lowest level of chromosomal heterozygosity and a restricted range of heterochromatin content confined to the lower end of the known distributional spectrum.
Similar content being viewed by others
References
Arnason U, Benirschke K, Mead JG, Nichols WW (1977) Banded karyotypes of three whales: Mesophodon europaeus, M. carlhubbsi and Balaenoptera acutorostrata. Hereditas 87:189–200
Arnason U, Lutley R, Sandholt B (1980) Banding studies on six killer whales: an account of C-band polymorphism and G-band patterns. Cytogenet. Cell Genet 28:71–78
Bannerjee SK, Kevan DKMcE (1960) A preliminary revision of the genus Atractomorpha Saussure 1862 (Orthoptera:Acridoidea:Pyrgomorphidae). Treubia 25:165–189
Buckton KE, O'Riordan ML, Jacobs PA, Robinson UA, Hill R, Evans HJ (1976) C and Q-band polymorphisms in the chromosomes of three human populations. Ann Hum Genet 40:99–112
Chatterjee C (1975) Constitutive heterochromatin in grasshoppers. Proc. 2nd All India Congr. Cytol. Genet. 1975. J Cytol Genet Congr suppl:48–53
Greilhuber J (1979) C-band distribution, DNA content and base composition in Adoxa moschatellina (Adoxaceae), a plant with cold sensitive chromosome segments. P1 Syst Evol 131:243–259
Greilhuber J, Speta F (1978) Quantitative analyses of C-banded karyotypes and systematics in the cultivated species of the Scilla siberica group (Liliaceae). P1 Syst Evol 129:63–109
John B (1981) Heterochromatin variation in natural populations. Chromosomes Today 7:128–137
John B, King M (1977) Heterochromatin variation in Cryptobothrus chrysophorus II. Patterns of C-banding. Chromosoma 65:59–79
John B, King M (1982) Meiotic effects of supernumerary heterochromatin in Heteropternis obscurella. Chromosoma 85:39–65
Kenton A (1978) Giemsa C-banding in Gibasis (Commelinaceae). Chromosoma 65:309–324
Kevan DKMcE, Chen Y-K (1969) A revised synopsis of the genus Atractomorpha Saussure, 1862 (Orthoptera, Pyrgomorphidae), with an account of the African aberrans group. Zool J Linn Soc 48:141–198
Key KHL, Kevan DKMcE (1980) A revision of the Australian Atractomorphini (Orthoptera: pyrgomorphidae). Aust J Zool 28:717–773
King M (1980) C-banding studies on Australian hylid frogs: secondary construction structure and the concept of euchromatic transformation. Chromosoma 80:191–217
King M, John B (1980) Regularities and restrictions governing C-band variation in acridoid grasshoppers. Chromosoma 76:123–150
Mandahl N (1978) Variation in C-stained chromosome regions in European hedgehogs (Insectivora, Mammalia). Hereditas 89:107–128
Marks GE, Schweizer D (1974) Giemsa banding: karyotype differences in some species of Anemone and in Hepatica mobilis. Chromosoma 44:405–416
Mascarello JT, Hseu TC (1976) Chromosome evolution in wood rats, genus Neotoma (Rodentia, Cricetidae). Evolution 30:152–169
Mengden GA (1981) Linear differentiation of the C-band pattern of the W chromosome in snakes and birds. Chromosome 83:275–287
Miklos GLG, Nankivell RN (1976) Telomeric satellite DNA functions in regulating recombination. Chromosoma 56:143–167
Nankivell RN (1976) Karyotype differences in the crenaticeps group of Atractomorpha (Orthoptera, Acridoidea, Pyrgomorphidae). Chromosoma 56:127–142
Rehn JAG (1953) The grasshoppers and locusts of Australia II. Family Acrididae (Subfamily Pyrgomorphinae). CSIRO Melbourne
Sannomiya M (1973) Cytogenetic studies on natural populations of grasshoppers with special reference to B-chromosomes II. Atractomorpha bedeli. Chromosoma 44:99–106
Vosa CG (1973) Heterochromatin recognition and analysis of chromosome variation in Scilla siberica. Chromosoma 43:269–278
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
John, B., King, M. Population cytogenetics of Atractomorpha similis . Chromosoma 88, 57–68 (1983). https://doi.org/10.1007/BF00329504
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00329504