Abstract
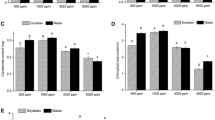
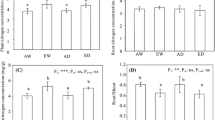
Abutilon theophrasti (C3) and Amaranthus retroflexus (C4), were grown from seed at four partial pressures of CO2: 15 Pa (below Pleistocene minimum), 27 Pa (pre-industrial), 35 Pa (current), and 70 Pa (future) in the Duke Phytotron under high light, high nutrient, and wellwatered conditions to evaluate their photosynthetic response to historic and future levels of CO2. Net photosynthesis at growth CO2 partial pressures increased with increasing CO2 for C3 plants, but not C4 plants. Net photosynthesis of Abutilon at 15 Pa CO2 was 70% less than that of plants grown at 35 Pa CO2, due to greater stomatal and biochemical limitations at 15 Pa CO2. Relative stomatal limitation (RSL) of Abutilon at 15 Pa CO2 was nearly 3 times greater than at 35 Pa CO2. A photosynthesis model was used to estimate ribulose-1,5-bisphosphate carboxylase (rubisco) activity (Vcmax), electron transport mediated RuBP regeneration capacity (J max), and phosphate regeneration capacity (PiRC) in Abutilon from net photosynthesis versus intercellular CO2 (A−C i) curves. All three component processes decreased by approximately 25% in Abutilon grown at 15 Pa compared with 35 Pa CO2. Abutilon grown at 15 Pa CO2 had significant reductions in total rubisco activity (25%), rubisco content (30%), activation state (29%), chlorophyll content (39%), N content (32%), and starch content (68%) compared with plants grown at 35 Pa CO2. Greater allocation to rubisco relative to light reaction components and concomitant decreases in J max and PiRC suggest co-regulation of biochemical processes occurred in Abutilon grown at 15 Pa CO2. There were no significant differences in photosynthesis or leaf properties in Abutilon grown at 27 Pa CO2 compared with 35 Pa CO2, suggesting that the rise in CO2 since the beginning of the industrial age has had little effect on the photosynthetic performance of Abutilon. For Amaranthus, limitations of photosynthesis were balanced between stomatal and biochemical factors such that net photosynthesis was similar in all CO2 treatments. Differences in photosynthetic response to growth over a wide range of CO2 partial pressures suggest changes in the relative performance of C3 and C4 annuals as atmospheric CO2 has fluctuated over geologic time.
Similar content being viewed by others
References
Ball TJ, Berry JA (1982) The Ci/Cs ratio: a basis for predicting stomatal control of photosynthesis. Carnegie Inst Wash Year Book 81:88–92
Barnola JM, Raynaud D, Korotkevich YS, Lorius C (1987) Vostok ice core provides 160,000 year record of atmospheric CO2. Nature 329:408–414
Bazzaz FA (1990) The response of natural ecosystems to the rising global CO2 levels. Annu Rev Ecol Syst 21:167–196
Bazzaz FA, Garbutt K (1988) The response of annuals in competitive neighborhoods: effects of elevated CO2. Ecology 69: 937–946
Byrd GT, Brown RH (1989) Environmental effects on photorespiration of C3−C4 species. I. Influence of CO2 and O2 during growth on photorespiratory characteristics and leaf anatomy. Plant Physiol 90:1022–1028
Campbell WJ, Allen LH Jr, Bowes G (1988) Effects of CO2 concentration on rubisco activity, amount and photosynthesis in soybean leaves. Plant Physiol 88:1310–1316
Collatz GJ, Ribas-Carbo M, Berry JA (1992) Coupled photosynthesis-stomatal conductance model for leaves of C4 plants. Aust J Plant Physiol 19:519–538
Curtis PS, Drake BG, Leadley PW, Arp WJ, Whigham DF (1989) Growth and senescence in plant communities exposed to elevated CO2 concentrations on an estuarine marsh. Oecologia 78:20–26
Dippery JK, Tissue DT, Thomas RB, Strain BR (1995) Effects of low and elevated CO2 on C3 and C4 annuals. I. Growth and biomass allocation. Oecologia 101:13–20
Ehleringer JR, Sage RF, Flanagan LB, Pearcy RW (1991) Climate change and the evolution of C4 photosynthesis. Trends Ecol Ecol 6:95–99
Evans JR, Terashima I (1987) Effects of nitrogen nutrition on electron transport components and phtosynthesis in spinach. Aust J Plant Physiol 14:59–68
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Field CB, Ball JT, Berry JA (1989) Photosynthesis: principles and field techniques. In: Pearcy RW, Ehleringer J, Mooney HA, Rundel PW (eds) Plant physiological ecology: field methods and instrumentation. Chapman and Hall, London, pp 209–253
Harley PC, Sharkey TD (1991) An improved model of C3 photosynthesis at high CO2: reversed O2 sensitivity explained by lack of glycerate re-entry into the chloroplast. Photosynth Res 27:169–178
Harley PC, Thomas RB, Reynolds JF, Strain BR (1992) Modelling photosynthesis of cotton grown in elevated CO2. Plant Cell Environ 15:271–282
Hellmers H, Giles LJ (1979) Carbon dioxide: critique I. In: Tibbitts TW, Kozlowski TT (eds) Controlled environment guidelines for plant research. Academic Press, New York, pp 229–234
Johnson HB, Polley HW, Mayeux HS (1993) Increasing CO2 and plant-plant interactions: effects on natural vegetation. Vegetatio 104/105:157–170
Jouzel J, Barkov NI, Barnola JM, Bender M, Chappellaz J, Genthon C, Kotlyakov VM, Lipenkov V, Lorius C, Petit JR, Raynaud D, Raisbeck G, Ritz C, Sowers T, Stievenard M, Yiou F, Yiou P (1993) Extending the Vostok ice-core record of palaeoclimate to the penultimate glacial period. Nature 364:407–412
Keeling CD, Bacastow RB, Carter AF, Piper SC, Whorf TP, Heimann M, Mook WG, Roeloffzen H (1989) A 3-dimensional model of atmospheric CO2 transport based on observed winds: 1. Analysis of observational data. In: Peterson DH (ed) Aspects of climate variability in the Pacific and the western Americas. Geophys Monogr 55:165–235
Leegood RC, Caemmerer S von (1989) Some relationships between contents of photosynthetic intermediates and the rate of photosynthetic carbon assimilation in leaves of Zea mays L. Planta 178:258–266
Lovelock JE, Whitfield M (1982) Life span of the biosphere. Nature 296:561–563
Lowther JR (1980) Use of a single sulfuric acid-hydrogen peroxide digest for the analysis of Pinus radiata needles. Comm Soil Sci Plant Anals 11:175–188
Morgan JA, Hunt HW, Monz CA, LeCain DR (1994) Consequences of long-term growth at various [CO2] and temperatures for leaf gas exchange of Pascopyrum smithii (C3) and Bouteloua gracilis (C4). Plant Cell Environ (in press)
Mousseau M, Enoch HZ (1989) Carbon dioxide enrichment reduces shoot growth in sweet chestnut seedlings (Castanea sativa Mill.). Plant Cell Environ 12:927–934
Overdieck D (1989) The effects of preindustrial and predicted future atmospheric CO2 concentration on Lyonia mariana L.D. Don. Funct Ecol 3:569–576
Pearcy RW, Bjorkman O (1983) Physiological effects. In: Lemon ER (ed) CO2 and plants: the response of plants to rising levels of atmospheric carbon dioxide. Westview, Boulder, pp 65–106
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochem Biophys Acta 975:384–394
Potvin C, Strain BR (1985) Photosynthetic response to growth temperature and CO2 enrichment in two species of C4 grasses. Can J Bot 63:483–487
Radoglou KM, Jarvis PG (1990) Effects of CO2 enrichment on four poplar clones. I. Growth and leaf anatomy. Ann Bot 65: 617–626
Ridley SM, Thornber JP, Bailey JL (1967) A study of the water soluble proteins of spinach beet chloroplasts with particular reference to fraction I protein. Biochem Biophys Acta 140: 62–79
Rowland-Bamford AJ, Baker JT, Allen LH Jr, Bowes G (1991) Acclimation of rice to changing atmospheric carbon dioxide concentration. Plant Cell Environ 14:577–583
Sage RF (1990) A model describing the regulation of ribulose-1,5-bisphosphate carboxylase, electron transport, and triose phosphate use in response to light intensity and CO2 in C3 plants. Plant Physiol 94:1728–1734
Sage RF (1994) Acclimation of photosynthesis to increasing atmospheric CO2: The gas exchange perspective. Photosynth Res 39:351–368
Sage RF, Reid CD (1992) Photosynthetic acclimation to sub-ambient CO2 (20 Pa) in the C3 annual Phaseolus vulgaris L. Photosynthetica 27:605–617
Sage RF, Sharkey TD (1987) The effect of temperature on the occurrence of O2 and CO2 insensitive photosynthesis in field grown plants. Plant Physiol 84:658–664
Sage RF, Sharkey TD, Seemann JR (1990) Regulation of ribulose-1,5-bisphosphate carboxylase activity in response to light intensity and CO2 in the C3 annuals Chenopodium album L. and Phaseolus vulgaris L. Plant Physiol 94:1735–1742
Sharkey TD (1985) Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. Bot Rev 51:53–105
Sharkey TD, Seemann JR, Berry JA (1986) Regulation of ribulose-1,5-bisphosphate carboxylase in response to changing partial pressure of O2 and light in Phaseolus vulgaris. Planta 176:415–424
Smith SD, Strain BR, Sharkey TD (1987) Effects of CO2 enrichment on four Great Basin grasses. Funct Ecol 1:139–143
Steer MW, Gunning BES, Graham TA, Carr DJ (1968) Isolation, properties, and structure of fraction I protein from Avena sativa L. Planta 79:254–267
Stitt M (1991) Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Environ 14: 741–762
Thomas JF, Harvey CN (1983) Leaf anatomy of four species grown under continuous CO2 enrichment. Bot Gaz 144: 303–309
Thomas RB, Strain BR (1991) Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiol 96:627–634
Tissue DT, Oechel WC (1987) Response of Eriophorum vaginatum to elevated CO2 and temperature in the Alaskan tussock tundra. Ecology 68:401–410
Tissue DT, Thomas RB, Strain BR (1993) Long-term effects of elevated CO2 and nutrients on photosynthesis and rubisco in loblolly pine seedlings. Plant Cell Environ 16:859–865
Wong SC (1979) Elevated atmospheric partial pressure of CO2 and plant growth. I. Interactions of nitrogen nutrition and photosynthetic capacity in C3 and C4 plants. Oecologia 44:68–74
Wray SM, Strain BR (1987) Competition in old-field perennials under CO2 enrichment. Ecology 68:1116–1120
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tissue, D.T., Griffin, K.L., Thomas, R.B. et al. Effects of low and elevated CO2 on C3 and C4 annuals. Oecologia 101, 21–28 (1995). https://doi.org/10.1007/BF00328895
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00328895