Summary
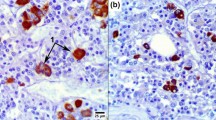
The morphological counterpart of the well-known age-dependent marked impairment of glucocorticoid secretion of rat adrenals was investigated by use of morphometric techniques. For this purpose 4-, 8-, 16- and 24-month-old rats were studied. Despite the notable lowering of both basal and ACTH-stimulated production of corticosterone by collagenase-dispersed inner adrenocortical cells, ACTH and corticosterone plasma concentrations displayed significant increases with ageing. Zona fasciculata (ZF) and zona reticularis (ZR) showed a notable hypertrophy in aged rats, which was due to rises in both the average volume and number of their parenchymal cells. The hypertrophy of ZF and ZR cells was in turn associated with increase in the volume of the mitochondrial compartment and proliferation of smooth endoplasmic reticulum, i.e., the two organelles involved in steroid-hormone synthesis. All these morphologic changes, conceivably due to the chronic exposure to high levels of circulating ACTH, are interpreted as a response enabling ZF and ZR to compensate for their age-dependent lowering in glucocorticoid secretion. Stereology also demonstrated that ZF and ZR cells underwent a striking age-related lipid-droplet repletion. Lipid droplets are the intracellular stores of cholesterol esters, the obligate precursors of steroid hormones in rats. This finding is in keeping with the contention that the mechanism underlying the age-dependent decline in rat-adrenal glucocorticoid secretion mainly involves impairments of the utilization of intracellular cholesterol previous to its intramitochondrial transformation to pregnenolone.
Similar content being viewed by others
References
Almeida H, Magalhaes MM, Magalhaes MC (1991) Cytoplasmic changes of zona reticularis cells of the adrenal cortex of the rat with aging. Colloque Franco-Iberique de Microscopie Eléctronique, Barcelona, Abstract Book, pp 82–83
Andersen JM, Dietschy JM (1976) Regulation of steroid synthesis in adrenal gland of the rat by both high and low density human lipoproteins. Biochem Biophys Res Commun 72:880–885
Andreis PG, Neri G, Cavallini L, Rebuffat P, Mazzocchi G, Nussdorfer GG (1989) Stereological and functional investigations on isolated adrenocortical cells. I. Adrenocortical cells of normal adult rats. J Submicrosc Cytol 21:357–365
Balasubramaniam S, Goldstein JL, Faust JR, Brunschede GY, Brown MS (1977) Lipoprotein-mediated regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesteryl ester metabolism in adrenal gland of the rat. J Biol Chem 252:1771–1779
Barnett JL, Cheeseman P, Douglas JK, Phillips JG (1974) Adrenal responsiveness in aging Brattleboro rats with hereditary diabetes insipidus. Age 3:189–193
Bell JBG, Gould RP, Hyatt PJ, Tait JF, Tait SAS (1979) Properties of rat adrenal zona reticularis cells: production and stimulation of certain steroids. J Endocrinol 83:435–447
Belloni AS, Neri G, Musajo FG, Andreis PG, Boscaro M, D'Agostino D, Rebuffat P, Boshier DP, Gottardo G, Mazzocchi G, Nussdorfer GG (1990) Investigations on the morphology and function of adrenocortical tissue regenerated from gland capsular fragments autotransplanted in the musculus gracilis of the rat. Endocrinology 126:3251–3262
Britton GW, Rotenberg S, Adelman RC (1975) Impaired regulation of corticosterone levels during fasting in aging rats. Biochem Biophys Res Commun 64:184–188
De Kosky ST, Scheff SW, Cotman CW (1984) Elevated corticosterone levels. A possible cause of reduced axon sprouting in aged animals. Neuroendocrinology 38:33–38
Elftheriou BE (1975) Changes with age in pituitary-adrenal responsiveness and reactivity to mild stress in mice. Gerontologia 20:224–230
Gwynne JT, Strauss JF III (1982) The role of lipoproteins in the steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev 3:299–329
Hess GD, Riegle GD (1970) Adrenocortical responsiveness to stress and ACTH in aging rats. J Gerontol 25:354–358
Hess GD, Riegle GD (1972) Effects of chronic ACTH stimulation on adrenocortical function in young and aged rats. Am J Physiol 222:1458–1461
Lacko AG, Carlile S, Kudchodkar BJ (1984) Evaluation of lipid and lipoprotein metabolism in rats as a model for studying age-related physiological changes. J Gerontol 39:513–520
Landfield PW, Waymire JC, Lynch G (1978) Hippocampal aging and adrenocorticoids: quantitative correlations. Science 202:1098–1101
Landfield PW, Sundberg DK, Smith MS, Eldridge JC, Morris M (1980) Mammalian aging: theoretical implications of changes in brain and endocrine systems during mid- and late life. Peptides 1 [Suppl 1]:185–196
Loud AV (1962) A method for the quantitative estimation of cytoplasmic structures. J Cell Biol 15:481–487
Malamed S, Carsia RV (1983) Aging of the rat adrenocortical cells: response to ACTH and cyclic AMP in vitro. J Gerontol 38:130–136
Miller WL (1988) Molecular biology of steroid hormone synthesis. Endocr Rev 9:295–318
Miller WL (1989) Regulation of mRNAs for human steroidogenic enzymes. Endocr Res 15:1–16
Moses HL, Davis WW, Rosenthal AS, Garren LD (1969) Adrenal cholesterol: localization by electron microscope autoradiography. Science 163:1203–1205
Nickerson PA, Feld LG, van Lieu JB (1979) Zona reticularis in aging spontaneously hypertensive rats: a quantitative ultrastructural study of 70- and 90-week-old animals. Am J Pathol 97:433–448
Nussdorfer GG (1986) Cytophysiology of the adrenal cortex. Int Rev Cytol 98:1–405
Nussdorfer GG, Mazzocchi G (1983) Long-term effects of ACTH on rat adrenocortical cells: a coupled stereological and enzymological study. J Steroid Biochem 19:1753–1756
Nussdorfer GG, Mazzocchi G (1987) Vasoactive intestinal peptide (VIP) stimulates aldosterone secretion by rat adrenal glands in vivo. J Steroid Biochem 26:203–205
Popplewell PY, Azhar S (1987) Effects of aging on cholesterol content and cholesterol-metabolizing enzymes in the rat adrenal gland. Endocrinology 121:64–73
Popplewell PY, Tsubokawa M, Ramachandran J, Azhar S (1986) Differential effects of aging on adrenocorticotropin receptors, adenosine 3′, 5′-monophosphate response, and corticosterone secretion in adrenocortical cells from Sprague-Dawley rats. Endocrinology 119:2206–2213
Popplewell PY, Butte J, Azhar S (1987) The influence of age on steroidogenic enzyme activities in the rat adrenal gland: enhanced expression of cholesterol side-chain cleavage activity. Endocrinology 120:2521–2528
Reaven E, Kostrna M, Ramachandran J, Azhar S (1988) Structure and function changes in rat adrenal glands during aging. Am J Physiol 255:E903-E911
Rees LH, Cook DM, Kendall JW, Allen CF, Kramer RM, Ratcliffe JG, Knight RA (1971) A radioimmunoassay for rat plasma ACTH. Endocrinology 89:254–261
Riegle GD (1973) Chronic stress effect on adrenocortical responsiveness in young and aged rats. Neuroendocrinology 11:1–10
Sandor T, Fazekas AG, Robinson BH (1976) The biosynthesis of corticosteroids throughout the vertebrates. In: Chester-Jones I, Henderson IW (eds) General, comparative and clinical endocrinology of the adrenal cortex, vol 1. Academic Press, London, pp 25–142
Sapolsky RM, Krey LC, Mc Ewen BS (1983) The adrenocortical stress response in the aged male rat: impairment of recovery from stress. Exp Gerontol 18:55–64
Shaposhnikov VM (1985) The ultrastructure of secretory cells of some endocrine glands in aging. Mech Ageing Devel 30:123–142
Shaposhnikov VM, Bezrukov VV (1985) Age changes in the ultrastructure and function of rat adrenal glands during stimulation of the hypothalamus. J Submicrosc Cytol 17:75–81
Simpson E, Mason JI, John ME, Zuber MX, Rodgers RJ, Waterman MR (1987) Regulation of the biosynthesis of steroidogenic enzymes. In: Mantero F, Vecsei P (eds) Corticosteroid and peptide hormones in hypertension. (Serono Symposia Publications, vol 39) Raven Press, New York, pp 1–9
Simpson ER, Lund J, Ahlgren R, Waterman M (1990) Regulation by cyclic AMP of the genes encoding steroidogenic enzymes: when the light finally shines. Mol Cell Endocrinol 70:C25-C28
Sippel WG, Bidlingmaier F, Becker H, Brünig T, Dörr H, Hahn H, Golder W, Hollmann G, Knorr D (1978) Simultaneous radioimmunoassay of plasma aldosterone, corticosterone, 11-deoxycorticosterone, progesterone, 17-hydroxyprogesterone, 11-deoxycortisol, cortisol and cortisone. J Steroid Biochem 9:63–74
Sonntag WE, Goliszek AG, Brodish A, Eldridge JC (1987) Diminished diurnal secretion of adrenocorticotropin (ACTH), but not corticosterone, in old male rats: possible relation to increased adrenal sensitivity to ACTH in vivo. Endocrinology 120:2308–2315
Szabo D, Dzsinich C, Okros I, Stark E (1970) The ultrastructure of the aged zona fasciculata under various stressing procedures. Exp Gerontol 5:335–337
Szalay KS (1981) Effects of pituitary intermediate lobe extract on steroid production by isolated zona glomerulosa and fasciculata cells. Acta Physiol Acad Sci Hung 57:225–231
Swinyard CA (1983) Methods for volumetric determination of fresh endocrine glands. Anat Rec 74:71–78
Tait JF, Tait SAS, Gould RP, Mee MSR (1974) The properties of adrenal zona glomerulosa cells after purification by gravitational sedimentation. Proc R Soc Lond [Biol] 185:375–407
Tang FT, Phillips JG (1978) Some age-related changes in pituitary-adrenal functions in the male laboratory rat. J Gerontol 33:377–382
Von Seebach HB, Lutzen L, Kreiner E, Ueberberg H, Pappritz G, Dhom G (1975) Morphology of aging in rat adrenals. Verh Dtsch Ges Pathol 59:414–418
Weibel ER (1979) Stereologic methods. 1. Practical methods for biological morphometry. Academic Press, London
Weibel ER, Paumgartner D (1978) Integrated stereological and biochemical studies on hepatocytic membranes. II. Correction of section thickness effect on volume and surface density estimates. J Cell Biol 77:584–597
Weiss L, Editor (1988) Cell and tissue biology. A textbook of histology. 6th edn. Urban & Schwarzenberg, Baltimore Munich, p 54
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rebuffat, P., Belloni, A.S., Rocco, S. et al. The effects of ageing on the morphology and function of the zonae fasciculata and reticularis of the rat adrenal cortex. Cell Tissue Res 270, 265–272 (1992). https://doi.org/10.1007/BF00328012
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00328012