Summary
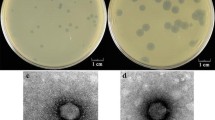
The gene coding for the bacterial plasminogen activator staphylokinase was cloned from the Staphylococcus aureus phage 42D, a serogroup F phage used for lysotyping, onto the standard Escherichia coli plasmid vector pACYC184. The coding and flanking sequences of the sak42D gene were largely identical to those of a sak gene cloned from the serologically different S. aureus phage SøC (Sako and Tsuchida 1983). Subcloning of a 2.5 kb phage 42D DNA fragment onto plasmid pGB3631 allowed the sak42D gene to be introduced into the gram-positive hosts Bacillus subtilis and Streptococcus sanguis. The sak42D gene was expressed and secreted most efficiently by B. subtilis cells (25 μg/ml of culture supernatant) reduced in exoprotease production. In this host expression and secretion of Sak was initiated at the early growth phase and continued through the logarithmic phase. Formation of Sak was, however, also observed with the other cloning hosts. The Sak elaborated by the heterologous hosts was serologically identical with authentic Sak derived from S. aureus.
Similar content being viewed by others
References
Backman K, Ptashne M, Gilbert W (1976) Construction of plasmids, carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci USA 73: 4714–4718
Behnke D (1981) Plasmid transformation of Streptococcus sanguis (Challis) occurs by circular and linear molecules. Mol Gen Genet 182: 490–497
Behnke D, Ferretti JJ (1980) Physical mapping of plasmid pDB101 — a potential vector plasmid for molecular cloning in streptococci. Plasmid 4: 130–138
Behnke D, Gilmore MS (1981) Location of antibiotic resistance determinants, copy control, and replication functions on the double-selective streptococcal cloning vector pGB301. Mol Gen Genet 184: 115–120
Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heynecker HL, Boyer HW, Crosa JH, Falkow S (1977) Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2: 95–113
Chang ACY, Cohen SN (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles from the P15A cryptic miniplasmid. J Bacteriol 134: 1141–1156
Christie R, Wilson H (1941) A test of staphylococcal fibrinolysin. Aust J Exp Biol Med Sci 19: 329
Dagert M, Ehrlich SD (1979) Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene 6: 23–28
Dubnau D, Davidoff-Abelson R (1971) Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol 56: 209–221
Fahnestock SR, Saunders CW, Guyer MS, Loefdahl S, Guss B, Uhlen M, Lindberg M (1986) Expression of the staphylococcal protein A gene in Bacillus subtilis by integration of the intact gene into the B. subtilis genome. J Bacteriol 165: 1011–1014
Fairweather N, Kennedy S, Foster TJ, Kehoe M, Dougan G (1983) Expression of a cloned Staphylococcus aureus alpha-hemolysin determinant in Bacillus subtilis and Staphylococcus aureus. Infect Immun 41: 1112–1117
Kawamura F, Doi RH (1984) Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J Bacteriol 160: 442–444
Kondo I, Fujise K (1977) Serotype B staphylococcal bacteriophage singly converting staphylokinase. Infect Immun 18: 266–272
Kondo I, Itoh S, Yoshizawa Y (1981) Staphylococcal phages mediating the lysogenic conversion of staphylokinase. Zentralb Bakteriol Mikrobiol Hyg [Suppl] 10: 357–362
Kovacevic S, Veal LE, Hsiung HM, Miller JR (1985) Secretion of staphylococcal nuclease by Bacillus subtilis. J Bacteriol 162: 521–528
Lack CH (1948) Staphylokinase: An activator of plasma protease. Nature 161: 559–560
Lee CY, Iandolo JJ (1985) Mechanism of bacteriophage conversion of lipase activity in Staphylococcus aureus. J Bacteriol 164: 288–293
Louvard D, Vannier C, Maroux S, Pages JM, Lazdunski C (1976) A quantitative immunochemical technique for evaluation of extent of integration of membrane proteins and of protein conformational changes and homologies. Anal Biochem 76: 83–94
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Moran CP, Lang N, LeGrice SFJ, Lee G, Stephens M, Sonenshein AL, Pero AL, Losick R (1982) Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet 186: 339–346
Peschke U, Beuck V, Bujard H, Gentz R, LeGrice S (1985) Efficient utilization of Escherichia coli transcriptional signals in Bacillus subtilis. J Mol Biol 186: 547–555
Priest FC (1977) Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev 41: 711–753
Rosenberg M, Court D (1979) Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet 13: 319–353
Sako T (1985) Overproduction of staphylokinase in Escherichia coli and its characterization. Eur J Biochem 149: 557–563
Sako T, Sawaki S, Sakurai T, Ito S, Yoshizawa Y, Kondo I (1983) Cloning and expression of the staphylokinase gene of Staphylococcus aureus in E. coli. Mol Gen Genet 190: 271–277
Sako T, Tsuchida N (1983) Nucleotide sequence of the staphylokinase gene from Staphylococcus aureus. Nucleic Acids Res 11: 7679–7693
Saksela O (1981) Radial caseinolysis in agarose: A simple method for detection of plasminogen activators in the presence of inhibitory substances and serum. Anal Biochem 111: 276–282
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Winkler KC, DeWaart J, Grootsen C, Zegers BJM, Tellier NF, Vertegt CD (1965) Lysogenic conversion of staphylococci to loss of beta-toxin. J Gen Microbiol 39: 321–333
Wong SL, Kawamura F, Doi RH (1986) Use of the Bacillus subtilis subtilisin signal peptide for efficient secretion of TEM betalactamase during growth. J Bacteriol 168: 1005–1009
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33: 103–119
Author information
Authors and Affiliations
Additional information
Communicated by W. Goebel
Rights and permissions
About this article
Cite this article
Behnke, D., Gerlach, D. Cloning and expression in Escherichia coli, Bacillus subtilis, and Streptococcus sanguis of a gene for staphylokinase — a bacterial plasminogen activator. Mol Gen Genet 210, 528–534 (1987). https://doi.org/10.1007/BF00327208
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00327208