Summary
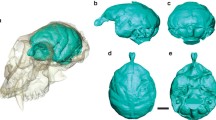
The brain of the La Plata dolphin, Pontoporia blainvillei, was studied with methods of quantitative morphology. The volumes and the progression indices of the main brain structures were determined and compared with corresponding data of other Cetacea, Insectivora and Primates.
In Pontoporia, encephalization and neocorticalization are clearly greater than in primitive (“basal”) Insectivora. The indices are in the lower part of the range for simian monkeys. The paleocortex is regressive in accordance with the total reduction of the olfactory bulb and olfactory tract. In contrast to the situation in primates, the septum, schizocortex and archicortex are not progressive in Pontoporia. The striatum and cerebellum are strongly progressive, corresponding to the efficiency and importance of the motor system in the three-dimensional habitat. The diencephalon, mesencephalon and medulla oblongata show considerable progression. Obviously, this is correlated with the extensive development of structures of the acoustic system.
The superficial correspondence of the brains of dolphins and primates in relative size and in the degree of gyrencephaly is rather a rough morphological convergence than a sign of functional equivalence. It is coupled to a strongly divergent development of the various functional systems in the two mammalian orders according to their specific evolution.
Similar content being viewed by others
References
Baron G (1974) Differential phylogenetic development of the acoustic nuclei among Chiroptera. Brain Behav Evol 9:7–40
Bauchot R (1978) Encephalization in vertebrates. Brain Behav Evol 15:1–18
Bauchot R, Stephan H (1968) Etude des modifications encéphaliques observées chez les insectivores adaptés à la recherche de nourriture en milieu aquatique. Mammalia 32:228–275
Chen P, Shao Z, Pilleri G (1980) Regression of the optic system in the Chang-jiang (Yangtze) finless porpoise (Neophocaena asiaeorientalis) as a result of lack of light. In: Pilleri G (ed) Investigations on Cetacea, Vol 11. Bern, pp 115–120
Dral ADG, Beumer L (1974) The anatomy of the eye of the ganges river dolphin Platanista gangetica (Roxburgh 1801). Z Säugetier 39:143–167
Filimonoff IN (1965) On the so-called rhinencephalon in the dolphin. J Hirnforsch 8:1–23
Frahm HD, Stephan H, Stephan M (1982) Comparison of brain structure volumes in Insectivora and Primates. I. Neocortex. J Hirnforsch 23:375–389
Gihr M, Pilleri G, Zhou K (1979) Cephalization of the chinese river dolphin Lipotes vexillifer (Platanistoidea, Lipotidae). In: Pilleri G (ed) Investigations on Cetacea. Bern, Vol 10, pp 257–274
Gruenberger HB (1970) On the cerebral anatomy of the amazon dolphin, Inia geoffrensis. In: Pilleri G (ed) Investigations on Cetacea, Vol 2. Bern, pp 129–144
Jacobs MS, McFarland WL, Morgane PJ (1979) The anatomy of the brain of the bottlenose dolphin (Tursiops truncatus). Rhinic lobe (rhinencephalon): The archicortex. Brain Res Bull 4, Suppl 1, pp 108
Jansen J (1952) On the whale brain with special reference to the weight of the brain of the fin whale. Norsk Hvalf Tid 9:480–486
Jansen J, Jansen JKS (1969) The nervous system of Cetacea. In: Andersen HT (ed) The biology of marine mammals. Academic Press New York-San Francisco-London, pp 175–252
Japha A (1911) Die Haare der Waltiere. Zool Jahrb 32:1–42
Kamiya T, Pirlot P (1980) Brain organization in Platanista gangetica. Sci Rep Whales Res Inst Tokyo 32:105–126
Kellogg R (1928) The history of whales—their adaptation to life in the water. Q Rev Biol 3:29–76, 174–208
Kraus C, Pilleri G (1969) Quantitative Untersuchungen über die Großhirnrinde der Cetaceen. In: Pilleri G (ed) Investigations on Cetacea, Vol 1, Bern, pp 127–150
Kruger L (1966) Specialized features of the Cetacean brain. In: Norris KS (ed) Whales, dolphins and porpoises. University of California Press Berkeley-Los Angeles, pp 232–254
Kruska D (1980) Domestikationsbedingte Hirngrößenänderungen bei Säugetieren. Z Zool Syst 18:161–195
Ladygina TF, Supin AY (1977) Localization of the projectional sensory areas in the cortex of the porpoise Tursiops truncatus. Zh Evol Biokhim Fiziol 13:712–718
Ladygina TF, Supin AY (1978) On the homology of the different regions of the brain's cortex of Cetacea and other mammals. In: Sokolov VE (ed) Morskiye Mlekopitayushchiye Resul'taty i Metodi Issledovaniyii. Izdatel'stvo Nauka, Moscow, pp 55–65
Ladygina TF, Mass AM, Supin AY (1978) Multiple sensory projections in the dolphin cerebral cortex. Zh Vyssh Nerv Deiat 28:1047–1053
Layne JN, Caldwell DK (1964) Behavior of the amazon dolphin, Inia geoffrensis (Blainville), in captivity. Zoologica NY 49:81–111
Lilly JC (1964) Animals in aquatic environments: Adaptation of mammals to the ocean. In: Handbook of physiology, chapt 46: Adaptation to the environment. American Physiological Society, Washington, pp 741–747
Mangold-Wirz K (1966) Cerebralisation and Ontogenesemodus bei Eutherien. Acta Anat (Basel) 63:449–508
Morgane PJ, Jacobs MS (1972) Comparative anatomy of the Cetacean nervous system. In: Harrison RJ (ed) Functional anatomy of marine mammals. Academic Press London-New York, pp 117–244
Morgane PJ, Jacobs MS, McFarland WL (1980) The anatomy of the brain of the bottlenose dolphin (Tursiops truncatus). Surface configurations of the telencephalon of the bottlenose dolphin with comparative anatomical observations in four other cetacean species. Brain Res Bull 5, Suppl 3:1–107
Norris KS (1964) Some problems of echolocation in Cetaceans. In: Tavolga WN (ed) Marine bioacoustics. Pergamon Press Oxford, pp 317–336
Oelschläger HA, Buhl EH (in press) Occurrence of an olfactory bulb in the early development of the harbor porpoise (Phocoena phocoena). In: Duncker H-R, Fleischer G (eds) Functional morphology of vertebrates. An International Symposium on Vertebrate Morphology, Gießen. Fischer Stuttgart
Pilleri G (1964) Morphologie des Gehirns des “Southern Right Whale”, Eubalaena australis Desmoulins 1822 (Cetacea, Mysticeti, Balaenidae). Acta Zool 46:245–272
Pilleri G (1966) Über die Anatomie des Gehirns des Gangesdelphins Platanista gangetica. Rev Suisse Zool 73:113–118
Pilleri G (1972) Cerebral anatomy of the Platanistidae (Platanista gangetica, Platanista indi, Pontoporia blainvillei, Inia geoffrensis). In: Pilleri G (ed) Investigations on Cetacea, Vol 4. Bern, pp 44–70
Pilleri G, Busnel RG (1969) Brain/body weight ratios in Delphinidae. Acta Anat (Basel) 73:92–97
Pilleri G, Chen P (1982) The brain of the chinese finless porpoise Neophocaena asiaeorientalis (Pilleri & Gihr 1972): I. Macroscopic anatomy. In: Pilleri G (ed) Investigations on Cetacea, Vol 13. Bern, pp 27–32
Pilleri G, Gihr M (1968) On the brain of the amazon dolphin, Inia geoffrensis de Blainville 1817 (Cetacea, Susuidae). Experientia 24:932–933
Pilleri G, Gihr M (1969a) Über adriatische Tursiops truncatus (Montagu 1821) und vergleichende Untersuchungen über mediterrane und atlantische Tümmler. In: Pilleri G (ed) Investigations on Cetacea, Vol 1. Bern, pp 66–73
Pilleri G, Gihr M (1969b) Zur Anatomie und Pathologie von Inia geoffrensis de Blainville 1817 (Cetacea, Susuidae) aus dem Beni, Bolivien. In: Pilleri G (ed) Investigations on Cetacea, Vol 1. Bern, pp 94–106
Pilleri G, Gihr M (1970a) Brain-body weight ratio of Platanista gangetica. In: Pilleri G (ed) Investigations on Cetacea, Vol 2. Bern, pp 79–82
Pilleri G, Gihr M (1970b) The central nervous system of the Mysticete and Odontocete whales. In: Pilleri G (ed) Investigations on Cetacea, Vol 2. Bern, pp 89–127
Pilleri G, Gihr M (1971) Brain-body weight ratio in Pontoporia blainvillei. In: Pilleri G (ed) Investigations on Cetacea, Vol 3, Part 1. Bern, pp 69–73
Pilleri G, Gihr M (1972) Contribution to the knowledge of the cetaceans of Pakistan with particular reference to the genera Neomeris, Sousa, Delphinus and Tursiops and description of a new chinese porpoise (Neomeris asiaeorientalis). In: Pilleri G (ed) Investigations on Cetacea, Vol 4. Bern, pp 107–162
Pilleri G, Gihr M (1976) On the embryology of Platanista gangetica. 1. Body proportions, external characteristics and radiological investigations. In: Pilleri G (ed) Investigations on Cetacea, Vol 7. Bern, pp 45–64
Pilleri G, Kraus C, Gihr M (1968) The structure of the cerebral cortex of the ganges dolphin Susu (Platanista) gangetica Lebeck 1801. Z Mikrosk Anat Forsch 79:373–388
Pirlot P, Kamiya T (1975) Comparison of ontogenetic brain growth in marine and coastal dolphins. Growth 39:507–524
Rice DW (1977) A list of the marine mammals of the world, third ed, NOAA Technical Report NMFS SSRF-771. United States Government Printing Office, Washington, III+15 pp
Ridgway SH, Flanigan NJ, McCormick JG (1966) Brain-spinal cord ratios in porpoises: possible correlations with intelligence and ecology. Psychon Sci 6:491–492
Starck D (1965) Die Neencephalisation (Die Evolution zum Menschenhirn). In: Heberer G (ed) Menschliche Abstammungslehre (Fortschritte der Anthropogenie 1863–1964). Fischer Stuttgart, pp 103–144
Starck D (1975) Neenkephalisation. In: Kurth G, Eibl-Eibesfeldt I (eds) Hominisation und Verhalten. Fischer Stuttgart, pp 201–233
Stephan H (1967) Zur Entwicklungshöhe der Insektivoren nach Merkmalen des Gehirns und die Definition der “Basalen Insektivoren”. Zool Anz 179:177–199
Stephan H (1972) Evolution of primate brains: a comparative anatomical investigation. In: Tuttle R (ed) The functional and evolutionary biology of primates. Aldine/Atherton Chicago, pp 155–174
Stephan H (1975) Allocortex. In: Bargmann W (ed) Handbuch der mikroskopischen Anatomie des Menschen Vol 4 (Nervensystem), Part 9. Springer, Berlin-Heidelberg-New York
Stephan H, Andy OJ (1964) Quantiative comparisons of brain structures from insectivores to primates. Am Zool 4:59–74
Stephan H, Andy OJ (1969) Quantitative comparative neuroanatomy of primates: an attempt at a phylogenetic interpretation. In: Albertson PD, Krauss M (eds) Comparative and evolutionary aspects of the vertebrate central nervous system. Ann N Y Acad Sci 167: 370–387
Stephan H, Kuhn H-J (1982) The brain of Micropotamogale lamottei Heim de Balsac 1954. Z Säugetier 47:129–142
Stephan H, Frahm H, Baron G (1981) New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol (Basel) 35:1–29
Stone H, Rebert CS (1970) Observations on trigeminal olfactory interactions. Brain Res 21:138–142
Supin AY, Mukhametov LM, Ladygina TF, Popov VV, Mass AM, Polyakova IG (1978) Electrophysiological studies of the dolphin's brain. In: Sokolov VE (ed) Izdatel'stvo Nauka, Moscow, pp 7–85
Swanson LW, Teyler TJ, Thompson RF (1982) Hippocampal long-term potentiation: mechanisms and implications for memory. Neurosci Res Program Bull 20
Waller GNH (1982) Retinal ultrastructure of the amazon river dolphin (Inia geoffrensis). Aquat Mamm 9:17–28
Warncke P (1908) Mitteilung neuer Gehirn-und Körpergewichtsbestimmungen bei Säugern, nebst Zusammenstellung der gesamten bisher beobachteten absoluten und relativen Gehirngewichte bei den verschiedenen Spezies. J Psych Neurol 13:355–403
Weber M (1897) Vorstudien über das Hirngewicht der Säugetiere. Festschrift C Gegenbaur, Vol 3. Engelmann Leipzig, pp 104–123
Zhou K, Pilleri G, Li Y (1979) Observations on the Baiji (Lipotes vexillifer) and the finless porpoise (Neophocaena asiaeorientalis) in the Changjiang (Yangtze) river between Nanjing and Taiyangzhou, with remarks on some physiological adaptations of the Baiji to its environment. In: Pilleri G (ed) Investigations on Cetacea, Vol 10. Bern, pp 109–120
Zvorykin VT (1963) Morphological substrate of ultrasonic and location properties in dolphin. Arch Anat Histol Embryol (Leningrad) 45:3–17
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schwerdtfeger, W.K., Oelschläger, H.A. & Stephan, H. Quantitative neuroanatomy of the brain of the La Plata dolphin, Pontoporia blainvillei . Anat Embryol 170, 11–19 (1984). https://doi.org/10.1007/BF00319453
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00319453