Summary
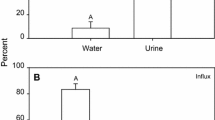
Exposure of adult brown bullheads Ictalurus nebulosus (120–450 g) to environmental hypercapnia (2% carbon dioxide in air) and subsequent recovery caused transient changes in whole body net sodium flux (J Na+net ) and net chloride flux (J Cl-net ) resulting largely from changes in whole body sodium influx (J Na+in ) and chloride influx (J Cl-in ). Scanning electron microscopy (SEM) revealed that the fractional area of chloride cells (CCs) on the interlamellar regions was reduced by 95% during environmental hypercapnia. During post-hypercapnic recovery, gill filament CC fractional area increased. The changes in J Cl-in during and after environmental hypercapnia were closely associated with the changes in CC fractional area while the changes in J Na+in did not correspond to the changes in CC fractional area. Transmission electron microscopy (TEM) supported the SEM observations of CC surface area changes and demonstrated that these changes were caused by covering/uncovering by adjacent pavement cells (PVCs). Lamellar and filament PVC microvilli density increased during hypercapnia while there was a subsequent reduction in the post-hypercapnic period. These data suggest that an important mechanism of acid-base regulation during hypercapnic acidosis is modification of the chloride cell-associated Cl-/HCO -3 exchange mechanism. We suggest that bullheads vary availability, and thus functional activity, of this transporter via reversible morphological alterations of the gill epithelium. The increase in density of PVC microvilli may be associated with sodium uptake and/or acidic equivalent excretion during acidosis.
Similar content being viewed by others
References
Avella M, Bornancin M (1989) A new analysis of ammonia and sodium transport through the gills of the freshwater rainbow trout (Salmo gairdneri). J Exp Biol 142:155–175
Avella M, Masoni A, Bornancin M, Mayer-Gostan N (1987) Gill morphology and sodium influx in the rainbow trout (Salmo gairdneri) acclimated to artificial freshwater environments. J Exp Zool 241:159–169
Bartels H (1989) Freeze-fracture study of the pavement cell in the lamprey gill epithelium. Analogy of membrane structure with the granular cell in the amphibian urinary bladder. Biol Cell 66:165–171
Cameron JN (1976) Branchial ion uptake in arctic grayling: resting values and the effects of acid-base disturbance. J Exp Biol 64:711–725
Cameron JN (1989) Acid-base homeostasis: past and present perspectives. Physiol Zool 62:845–865
Cameron JN, Iwama GK (1987) Compensation of progressive hypercapnia in channel catfish and blue crabs. J Exp Biol 133:183–197
Ehrenfeld J, Lacoste I, Garcia-Romeu F, Harvey B (1990) Interdependence of Na+ and H+ transport in frog skin. In: Truchot JP, Lahlou B (ed) Animal nutrition and transport processes. 2. Transport, respiration, and excretion: comparative and environmental aspects. Comp Physiol 6:152–170
Foskett JK, Scheffey C (1982) The chloride cell: definitive identification as the salt-secretory cell in teleosts. Science 215:164–166
Girard JP, Payan P (1980) Ion exchanges through respiratory and chloride cells in freshwater- and seawater-adapted teleosteans. Am J Physiol 7:R260-R268
Goss GG, Wood CM (1990a) Na+ and Cl- uptake kinetics, diffusive effluxes, and acidic equivalent fluxes across the gills of rainbow trout. I. Responses to environmental hyperoxia. J Exp Biol 152:521–547
Goss GG, Wood CM (1990b) Na+ and Cl- uptake kinetics, diffusive effluxes, and acidic equivalent fluxes across the gills of rainbow trout. II. Responses to bicarbonate infusion. J Exp Biol 152:549–571
Goss GG, Wood CM (1991) Two-substrate kinetics analysis: a novel approach linking ionic and acid-base transport in freshwater trout. J Comp Physiol[B] 161:635–646
Hughes G (1979) Scanning electron microscopy of the respiratory surfaces of trout gills. J Zool 188:443–453
Keys AB, Wilmer EN (1932) “Chloride secreting cells” in the gills of fishes with special reference to the common eel. J Physiol London 76:368–378
Kristensen P, Ussing HH (1985) Epithelia organization. In: Seldin W, Giesbisch G (eds) The kidney: physiology and pathophysiology. Raven Press, New York, pp 173–188
Krogh A (1938) The active absorption of ions in some freshwater animals. Zeit Vergl Physiol 25:335–350
Laurent PL (1984) Gill internal morphology. In: Hoar WS, Randall DJ (eds) Fish physiology, vol XA. Academic Press, New York, pp 73–183
Laurent PL (1989) Gill structure and function: fish. In: Wood SC (ed) Comparative pulmonary physiology. Dekker, New York, pp 69–120
Laurent PL, Dunel S (1980) Morphology of gill epithelia in fish. Am J Physiol 238:R147-R159
Laurent PL, Hebibi N (1989) Gill morphometry and fish osmoregulation. Can J Zool 67:3055–3063
Laurent PL, Perry SF (1990) Effects of cortisol on gill chloride cell morphology and ionic uptake in the freshwater trout, Salmo gairdneri. Cell Tissue Res 259:429–442
Laurent PL, Höbe H, Dunel-Erb S (1985) The role of environmental sodium chloride relative to calcium in gill morphology of freshwater salmonid fish. Cell Tissue Res 240:675–692
Lindemann B, Van Driessche W (1978) The mechanism of Na+ uptake through Na+ selective channels in the epithelium of frog skin. In: Hoffman JF (ed) Membrane transport processes, vol 1. Raven Press, New York, pp 155–178
Madsen KM, Tisher CC (1985) Structure-function relationships in H+-secreting epithelia. Federation Proc 44:2704–2709
Maetz J (1974) Aspects of adaptation to hypo-osmotic and hyperosmotic environments. In: Malins DC, Sargent JR (eds) Biochemical and biophysical perspectives in marine biology, vol 1. Academic Press, New York, pp 1–167
McDonald DG, Wood CM (1981) Branchial and renal acid and ion fluxes in the rainbow trout, Salmo gairdneri, at low environmental pH. J Exp Biol 93:101–118
McDonald DG, Tang Y, Boutilier RG (1989) Regulation of acid and ion transfer across the gills of fish. Can J Zool 67:3046–3054
Olson KR, Fromm PO (1973) A scanning electron microscopic study of secondary lamellae and chloride cells of rainbow trout (Salmo gairdneri). Z Zellforsch Mikrosk Anat 143:439–449
Perry SF, Laurent PL (1989) Adaptational responses of rainbow trout to lowered external NaCl concentration: contribution of the branchial chloride cell. J Exp Biol 147:168
Perry SF, Malone S, Ewing D (1987a) Hypercapnic acidosis in the rainbow trout (Salmo gairdneri). I. Branchial ion fluxes and blood acid-base status. Can J Zool 65:888–895
Perry SF, Malone S, Ewing D (1987b) Hypercapnic acidosis in the rainbow trout (Salmo gairderni). II. Renal ionic fluxes. Can J Zool 65:896–902
Potts WT (1984) Transepithelial potentials in fish gills. In: Hoar WS, Randall DJ (eds) Fish physiology, vol XB. Academic Press, New York, pp 39–63
Shaw J (1959) The absorption of sodium ions by the crayfish Astacus pallipes (Lebeboullet). I. The effect of external and internal sodium concentration. J Exp Biol 36:126–144
Stewart PA (1983) Modern quantitative acid-base chemistry. Can J Physiol Pharmacol 61:1444–1461
Weibel ER, Kistler GS, Sherle WF (1966) Practical stereological methods for morphometric cytology. J Cell Biol 30:23–38
Wood CM (1988) Acid-base and ionic exchanges at gills and kidney after exhaustive exercise in the rainbow trout. J Exp Biol 136:461–481
Wood CM, Goss GG (1990) Kinetic analysis of the relationships between ion exchange and acid-base regulation at the gills of freshwater fish. In: Truchot JP, Lahlou B (eds) Animal nutrition and transport processes. 2. Transport, respiration and excretion: comparative and environmental aspects. Comp Physiol 6:119–136
Wood CM, Wheatly M, Höbe H (1984) The mechanisms of acid-base and ionoregulation in the freshwater rainbow trout during environmental hyperoxia and subsequent normoxia. III. Branchial exchanges. Respir Physiol 55:175–192
Zall DM, Fisher MD, Garner QM (1956) Photometric determination of chloride in water. Anal Chem 28:1665–1678
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goss, G.G., Laurent, P. & Perry, S.F. Evidence for a morphological component in acid-base regulation during environmental hypercapnia in the brown bullhead (Ictalurus nebulosus). Cell Tissue Res 268, 539–552 (1992). https://doi.org/10.1007/BF00319161
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00319161