Summary
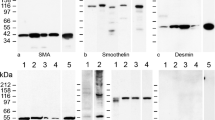
The histochemical demonstration of alkaline phosphatase (AP) activity and localization of smooth muscle myosin (SMM), F-actin, and desmin were carried out on frozen sections of testes and ovaries from 15-day-old fetal to newborn rats. The presence of immunocytochemically localized SMM and desmin was confirmed by Western blot analysis of proteins from isolated gonads. The development of smooth muscle cells was predominant in the testis. The first SMM-positive cells with an increasing intensity for F-actin and desmin appeared in the testicular tunica albuginea and around the testicular cords by the age of 16 days. A continuous layer of SMM- and F-actin-positive (but not uniformly desmin-positive) myoid cells was detected in the newborn testis. In the early gonads and in the newborn ovary, a majority of the interstitial cells expressed desmin, indicating that, in undifferentiated tissues, non-myogenic cells may also express desmin. During fetal development, male and female gonocytes showed a decrease in F-actin content but retained their high AP activity. In the cortex of the newborn rat ovary, the observed high AP activity and the presence of desmin may be associated with the postnatal histogenesis of the follicles. The presence of SMM-containing cells in the hilus of the ovary may be required for the demarcation of the ovary from the mesonephros by the constriction of the mesovarium. The occurrence of SMM-positive cells predominantly in male fetuses suggests that the development of the contractile cells in the fetal testis may be induced by testicular androgens.
Similar content being viewed by others
References
Amsterdam A, Lindner HR, Gröschel-Stewart U (1977) Localization of actin and myosin in the rat oocyte and follicular wall by immunofluorescence. Anat Rec 187:311–328
Anthony CT, Skinner MK (1989) Cytochemical and biochemical characterization of testicular peritubular myoid cells. Biol Reprod 40:811–823
Bressler RS, Ross MH (1972) Differentiation of peritubular myoid cells of the testis: effects of intratesticular implantation of newborn mouse testis into normal and hypophysectomized adults. Biol Reprod 6:148–159
Chapin RE, Phelps JL, Miller BE, Gray TJB (1987) Alkaline phosphatase histochemistry discriminates peritubular cells in primary rat testicular cell culture. J Androl 8:155–161
Cunha GR, Shannon JM, Neubauer BL, Sawyr LM, Fujii H, Taguchi O, Chung LWK (1981) Mesenchymal-epithelial interactions in sex differentiation. Hum Genet 58:68–77
Döhler KD, Wuttke W (1974) Serum LH, FSH, prolactin and progesterone from birth to puberty in female and male rats. Endocrinology 94:1003–1008
Dürnberger H, Kratochwil K (1980) Specificity of tissue interaction and origin of mesenchymal cells in the androgen response of the embryonic mammary gland. Cell 19:465–471
Feldman SC, Bloch E (1978) Developmental pattern of testosterone synthesis by fetal rat testes in response to luteinizing hormone. Endocrinology 102:999–1007
Ford DH, Hirschman A (1955) Alkaline phosphatase activity in the ovaries of immature and maturing albino rats. Anat Rec 121:531–547
Franke WW, Grund C, Schmid E (1979) Intermediate-sized filaments present in Sertoli cells are of the vimentin type. Eur J Cell Biol 19:269–275
Fröjdman K, Paranko J, Kuopio T, Pelliniemi LJ (1989) Structural proteins in sexual differentiation of embryonic gonads. Int J Dev Biol 33:99–103
Gomori G (1941) The distribution of phosphatase in normal organs and tissues. J Cell Comp Physiol 17:71–84
Hargrove JL, Seeley RR, Johnson JM, Ellis LC (1973) Prostaglandin-like substances: initiation and maintenance of rabbit testicular contractions in vitro. Proc Soc Exp Biol Med 142:205–209
Kormano M (1967) Dye permeability and alkaline phosphatase activity of testicular capillaries in the postnatal rat. Histochemie 9:327–338
Kormano M, Hovatta O (1972) Contractility and histochemistry of the myoid cell layer during postnatal development. Z Anat Entwicklungsgesch 137:239–248
Kornblatt MJ, Klugerman A, Nagy F (1983) Characterization and localization of alkaline phosphatase activity in rat testes. Biol Reprod 29:157–164
Kuopio T, Paranko J, Pelliniemi LJ (1989) Basement membrane and epithelial features of fetal-type Leydig cells in rat and human testis. Differentiation 40:198–206
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lording DW, Kretser DM de (1972) Comparative ultrastructural and histochemical studies of the interstitial cells of the rat testis during fetal and postnatal development. J Reprod Fertil 29:261–269
Magre S, Jost A (1980) The initial phases of testicular organogenesis in the rat. An electron microscopy study. Arch Anat Microsc Morphol Exp 69:297–318
McAlpine RJ (1955) Alkaline glycerophosphatase in the developing adrenal, gonads, and reproductive tract of the white rat. Anat Rec 121:407–408
McLean IW, Nakane PK (1974) Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem 22:1077–1083
Merchant-Larios H (1976) The onset of testicular differentiation in the rat: an ultrastructural study. Am J Anat 145:319–330
Merchant-Larios H, Mendlovic F, Alvarez-Buylla A (1985) Characterization of alkaline phosphatase from primordial germ cells and ontogenesis of this enzyme in the mouse. Differentiation 29:145–151
Niemi M, Ikonen M (1965) Primordial germ cells in foetal and postnatal human testis. Ann Med Exp Fenn 43:23–28
Niemi M, Setchell BP (1986) Gamma glutamyl transpeptidase in the vasculature of the rat testis. Biol Reprod 35:385–391
Osborn M, Weber K (1983) Biology of disease. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest 48:372–394
Palombi F, DiCarlo C (1988) Alkaline phosphatase is a marker for myoid cells in cultures of rat peritubular and tubular tissue. Biol Reprod 39:1101–1109
Paranko J (1987) Expression of type I and III collagen during morphogenesis of fetal rat testis and ovary. Anat Rec 219:91–101
Paranko J, Pelliniemi LJ, Dym M, Fujiwara K, Pollard T (1981) Postnatal development of myosin containing cells in the male rat reproductive tract. In: Byskov AG, Peters H (eds) Development and function of reproductive organs. Excerpta Medica, Amsterdam-Oxford-Princeton, pp 191–198
Paranko J, Pelliniemi LJ, Vaheri A, Foidart J-M, Lakkala-Paranko T (1983) Morphogenesis and fibronectin in sexual differentiation of rat embryonic gonads. Differentiation 23 (S):72–81
Paranko J, Kallajoki M, Pelliniemi LJ, Lehto V-P, Virtanen I (1986) Transient coexpression of cytokeratin and vimentin in differentiating rat Sertoli cells. Dev Biol 117:35–44
Picon R (1976) Testosterone secretion by foetal rat testes in vitro. J Endocrinol 71:231–238
Pollard TD (1981) Cytoplasmic contractile proteins. J Cell Biol 91:156S-165S
Pollard TD, Fujiwara K, Niederman R, Maupin-Szamier P (1976) Actin, myosin and associated proteins. In: Goldman R, Pollard T, Rosenbaum J (eds) Cell motility, book B. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 689–724
Redi CA, Hilscher B, Winking H (1983) Stage-dependent enzymatic activities in spermatogenesis of mice with the standard karyotype and of chromosomal variants with impaired fertility. Andrologia 15:322–330
Roosen-Runge EC, Anderson D (1959) The development of the interstitial cells in the testis of the albino rat. Acta Anat 37:125–137
Skinner MK, Fritz IB (1985) Androgen stimulation of Sertoli cell function is enhanced by peritubular cells. Mol Cell Endocrinol 40:115–122
Sternberger LA (1979) Immunocytochemistry, 2nd edn. Wiley, New York
Tung PS, Fritz IB (1980) Interactions of Sertoli cells and myoid cells in vitro. Biol Reprod 23:207–217
Tung PS, Fritz IB (1987) Morphogenetic restructuring and formation of basement membranes by Sertoli cells and testis peritubular cells in co-culture: inhibition of the morphogenesis cascade by cyclic AMP derivatives and by blocking direct cell contact. Dev Biol 120:139–153
Tung SP, Fritz IB (1990) Characterization of rat testicular peritubular myoid cells in culture: α-smooth muscle isoactin is a specific differentiation marker. Biol Reprod 42:351–365
Verhoeven G (1980) Androgen receptor in cultured interstitial cells derived from immature rat testis. J Steroid Biochem 13:469–474
Virtanen I, Kallajoki M, Närvänen O, Paranko J, Thornell L-E, Miettinen M, Lehto V-P (1986) Peritubular myoid cells of human and rat testis are smooth muscle cells that contain desmintype intermediate filaments. Anat Rec 215:10–20
Vogl AW (1989) Distribution and function of organized concentrations of actin filaments in mammalian spermatogenic cells and Sertoli cells. Int Rev Cytol 119:1–56
Wang Y (1991) Dynamics of the cytoskeleton in live cells. Curr Op Cell Biol 3:27–32
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paranko, J., Pelliniemi, L.J. Differentiation of smooth muscle cells in the fetal rat testis and ovary: localization of alkaline phosphatase, smooth muscle myosin, F-actin, and desmin. Cell Tissue Res 268, 521–530 (1992). https://doi.org/10.1007/BF00319159
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00319159