Summary
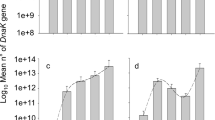
Exploiters of short-lived plants have evolved strategies in response to physiological changes that occur during the development of their hosts. The ability to adapt to host quality changes is necessary particularly if the mobility of an animal is poor or risk-constrained. In the plant-aphid system Centaurea jaceae-Uroleucon jaceae, the responses of the aphid to the seasonal changes in its host plant grown in poor and good quality soil were investigated. Coarse- and fine-tuned physiological reactions were observed in discrete aphid generations which were reared on plants grown either in a growth chamber or in a greenhouse. The number of ovarioles and developmental time depended on extrinsic factors (length of photoperiod) not directly related to plant quality. The reproductive investment of the aphids was also independent of their total dry weight. However, within the gonadal system high correlations were found at the embryonic level (e.g. the number of sclerotized embryos or the length of the oldest embryos per ovariole were highly correlated with gonadal dry weight). Aphids which were living on the 4-leaf stage of high-quality host plants showed a significantly higher investment in their gonads than aphids feeding on senescenting hosts. Factor analysis corroborated that aphids reared on poor-quality hosts revealed no grouping of variables measured, whereas those which were reared on high-quality plants showed clustering in respect of somatic (tibia length, dry weight of soma) and gonadal (number of sclerotized and unsclerotized embryos, length of the oldest embryos, ovariole number, gonad dry weight) factors. Three different levels of adaptive response to plant quality are distinguished: individual response to host quality, maternal influences on offspring and response to changes not specific to habitat (photoperiod). These different levels of regulation are thought to enable the aphids to adapt to a host of a given nutritional quality and anticipate deteriorating habitat quality simultaneously. It is concluded that physiological constraints in aphids are only revealed when aphids are exposed to severe nutritional stress for several generations.
Similar content being viewed by others
References
Börner C (1952) Europae Centralis Aphids. Die Blattläuse Mitteleuropas. Mitteilung Thüringer Botanischen Gesellschaft. Beiheft 3, Weimar 484
Brough CN, Dixon AFG (1990) The effects of starvation on development and reproductive potential of apterous virginoparae of the vetch aphid, Megoura viciae. Entomol Exp App 55: 41–45
Chesson P, Rosenzweig M (1991) Behavior, heterogeneity and the dynamics of interacting species. Ecology 72: 1187–1195
Dillwith JW, Berberet RC, Bergman DK, Neese PA, Edwards RM, McNew RW (1991) Plant biochemistry and aphid populations: Studies on the spotted alfalfa aphid Therioaphis maculata. Arch Insect Biochem Physiol 17: 235–251
Dixon AFG (1985) Structure of aphid populations. Annu Rev Entomol 30: 155–174
Dixon AFG (1987) Parthenogenetic reproduction and rate of increase in aphids. In: Minks AK, Harrewijin P (eds) Aphids, their biology, natural enemies and control, Vol. 2B, Elsevier, Amsterdam, pp 269–287
Dixon AFG, Dharma TR (1980a) Number of ovarioles and fecundity in the black bean aphid, Aphis fabae. Entomol Exp Appl 28: 1–14
Dixon AFG, Dharma TR (1980b) Spreading of the risk in developmental mortality: size, fecundity and reproductive rate in the black bean aphid. Entomol Exp Appl 28: 301–312
Fereres A, Gutierrez C, Del Estal P, Castanera P (1988) Impact of the english grain aphid Sitobion avenae (F.) (Homoptera: Aphididae), on the yield of wheat plants subjected to water deficits. Environ Entomol 17: 596–602
Grüber K, Dixon AFG (1988) The effect of nutrient stress on development and reproduction in an aphid. Entomol Exp Appl 47: 23–30
Jansson RK, Smilowitz Z (1986) Influence of nitrogen on population parameters of potato insects: abundance, population growth and within plant distribution of the green peach aphid, Myzus persicae (Homoptera: Aphididae). Environ Entomol 15: 49–55
James FC, McCulloch CE (1990) Multivariate analysis in ecology and systematics: panacea or pandora's box? Annu Rev Ecol Syst 21: 129–166
Kennedy JS (1958) Physiological condition of the host plant and susceptibility to aphid attack. Entomol Exp Appl 1: 50–65
Kouamé KL, Mackauer M (1992) Influence of starvation on development and reproduction in apterous virginoparae of the Pea Aphid Acyrthosiphon pisum. Can Ent 124: 87–95
Leather SR (1987) Generation specific trends in aphid life history parameters. J Appl Entomol 104: 278–284
Leather SR, Dixon AFG (1981) The effect of cereal growth stage and feeding site on the reproductive activity of the bird-cherry aphid, Rhopalosiphum padi. Ann Appl Biol 97: 135–141
Leather SR, Wellings PW (1981) Ovariole number and fecundity in aphids. Entomol Gen Appl 30: 128–133
Linke F, Baur F (1970) Metereologisches Taschenbuch, Bd.II. Akademische Verlagsgesellschaft, Leipzig
Mittler TE (1958) Studies on the feeding and nutrition of Tuberolachnus salignus (Gmelin) (Homoptera, Aphididae). II. The nitrogen and sugar composition of ingested phloem sap and excreted honeydew. J Exp Biol 35: 74–84
Mosbacher GC (1963) Über die Nahrungswahl bei Dactynotus Raf. (Aphididae) I. Die Wirtsspektren der Gruppe D. jaceae (L.) s. lat und D. cichorii (Koch) s. lat. Z Angew Entomol 51: 377–428
Röber S, Schaller B (1985) Pflanzenernährung im Gartenbau. Handbuch des Erwerbsgärtners. Ulmer Stuttgart
Roitberg B, Mangel M, Tourigny G (1990) The density dependence of parasitism by Tephritid fruit flies. Ecology 71: 1871–1885
Sequeira R, Mackauer M (1992) Covariance of adult size and development time in relation to host size in the parasitoid wasp Aphidius ervi. Evol Ecol 6: 34–44
Sokal R, Rohlf J (1981) Biometry, 2nd edn. Freeman and Company, San Francisco
Stadler B (1989) Untersuchungen zur Populationsökologie von Uroleucon jaceae (L.) (Homoptera, Aphididae) an Centaureen in Oberfranken. Diploma thesis, University of Bayreuth
Stadler B (1990) Relationships between host plant quality and reproductive investment in Uroleucon jaceae (L.) (Aphididae). Acta Phytopathol Entomol Hung 25: 177–183
Van Emden HF, Bashford MA (1971) The performance of Brecivoryne brassicae and Myzus persicae in relation to plant age and leaf amino acids. Entomol Exp Appl 14: 349–360
Walters KFA, Dixon AFG (1983) Migratory urge and reproductive investment in aphids: Variation within clones. Oecologia 58: 70–75
Walters KFA, Brough C, Dixon AFG (1988) Habitat quality and reproductive investment in aphids. Ecol Entomol 13: 337–345
Ward SA, Dixon AFG (1982) Selective resorption of aphid embryos and habitat changes relative to life-span. J Anim Ecol 51: 859–864
Ward SA, Wellings PW, Dixon AFG (1983) The effect of reproductive investment on pre-reproductive mortality in aphids. J Anim Ecol 52: 305–313
Weibull J (1987) Seasonal changes in free amino acids of oat and barley phloem sap in relation to plant growth stage and growth of Rhopalosiphum padi. Ann Appl Biol 111: 729–737
Wellings PW, Leather SR, Dixon AFG (1980) Seasonal variation in reproductive potential: a programmed feature of aphid life cycles. J Anim Ecol 49: 975–985
Wiktelius S, Chiverton PA (1985) Ovariole number and fecundity for the two emigrating generations of the bird cherry-oat aphid (Rhopalosiphum padi) in Sweden. Ecol Entomol 10: 349–355
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stadler, B. Physiological responses of Uroleucon jaceae (L.) to seasonal changes in the quality of its host plant Centaurea jacea L.: multilevel control of adaptations to the life cycle of the host. Oecologia 91, 273–280 (1992). https://doi.org/10.1007/BF00317796
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317796