Summary
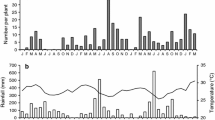
We studied interactions among collards, Brassica oleracea var. acephala, the diamondback moth (DBM), Plutella xylostella (Lepidoptera: Yponomeutidae) and its parasitoid Diadegma insulare (Hymenoptera: Ichneumonidae) by manipulating plant nitrogen (N) concentrations in field and laboratory experiments. Parasitoid abundance strongly reflected DBM abundance and was related to total leaf N. Parasitism rates were high (70.7%) and density-independent. Wasp sex ratios varied markedly (3–93% female) in response to the herbivores, the plants, or both. Higher proportions of female wasps emerged from DBM larvae on plants with high leaf N than on unfertilized plants. More female wasps also emerged from larvae parasitized as larger instars. We suggest that wasps have the potential to control DBM populations through long-term numerical responses mediated by variable sex ratios.
Similar content being viewed by others
References
Allen SE (ed) (1974) Chemical Analysis of Ecological Materials. Wiley, New York
Anderson RM, May RM (1978) Regulation and stability of hostparasite interactions. I. Regulatory processes. J Anim Ecol 47:219–247
Barbosa P (1988) Natural enemies and herbivore-plant interactions: influence of plant allelochemicals and host specificity. In: Barbosa P, Letourneau DK (eds) Novel Aspects of Insect-Plant Interactions. Wiley, NY, pp 201–229
Barbosa P, Saunders JA (1985) Plant allelochemicals: linkages between herbivores and their natural enemies. In: Cooper-Driver GA, Swain T, Conn EE (eds) Chemically Mediated Interactions between Plants and other Organisms, Plenum, NY, pp 107–137
Bouletreau M (1976) Influence de la photoperiode subie par les adultes sur la sex ratio de la descendance chez Pteromalus puparum (Hymenoptera; Chalcididae). Entomol Exp Appl 19:197–204
Bull JJ (1983) Evolution of Sex Determining Mechanisms. Benjamin-Cummings, Menlo Park CA
Campbell BC, Duffey SF (1979) Tomatine and parasitic wasps: potential incompatibility of plant antibiosis with biological control. Science 20:700–702
Campbell BC, Duffey SF (1981) Alleviation of tomatine induced toxicity to the parasitoid, Hyposoter exiguae by phytosterols in the diet of the host, Heliothis zea. J Chem Ecol 7:927–946
Charnov EL (1979) The genetical evolution of patterns of sexuality: Darwinian fitness. Am Nat 113:465–480
Charnov EL (1982) The Theory of Sex Allocation. Princeton University Press, Princeton NJ
Charnov EL, Skinner SW (1984) Evolution of host selection and clutch size in parasitoid wasps. Florida Entomol 67:5–21
Charnov EL, Hartogh RL, Jones WT, Assem J van den (1981) Sex ratio evolution in a variable environment. Nature 289:27–33
Chua TH, Ooi PAC (1986) Evaluation of three parasites in the biological control of diamondback moth in the Cameron Highlands, Malaysia. In: Talekar NS (ed), Diamondback Moth Management. Proc. First Intl Workshop, Asian Vegetable Research and Development Center. Shan-hua, Taiwan, pp 173–184
Clarke C (1984) Upsets in the sex-ratio of some Lepidoptera. In: Vane-Wright RI, Ackery PR (eds) The Biology of Butterflies. Academic Press, Orlando FL, pp 255–258
Comins HN, Wellings PW (1985) Density-related parasitoid sexratio: influence on host-parasitoid dynamics. J Anim Ecol 54:583–594
Compton SJ, Jones CG (1985) Mechanism of dye response and interface in the Bradford protein assay. Anal Biochem 151:369–374
Fischlin A, Baltensweiler W (1979) Systems analysis of the larch bud moth system. Part I: The larch-larch bud moth relationship. Mitt Schweiz Entomol Ges 52:273–289
Goodwin S (1979) Changes in numbers in the parasitoid complex associated with the diamond-back moth, Plutella xylostella (L.) (Lepidoptera). Aust J Zool 27:981–989
Hamilton WD (1967) Extraordinary sex ratios. Science 156:477–488
Harcourt DG (1963) Mortality factors in the population dynamics of the diamondback moth, Plutella maculipennis (Curt.) (Lepidoptera: Plutellidae). Mem Entomol Soc Canada 32:55–66
Harcourt DG (1986) Population dynamics of the diamondback moth in southern Ontario. In: Talekar NS (ed), Diamondback Moth Management. Proc. First Intl Workshop, Asian Vegetable Research and Development Center. Shan-hua, Taiwan, pp 1–15
Hassell MP (1978) The Dynamics of Arthropod Predator-Prey Systems. Princeton University Press, Princeton, NJ
Hassell MP (1986) Parasitoids and population regulation. In: Waage J, Greathead D (eds). Insect Parasitoids. Academic Press, Orlando FL, pp 201–224
Hassell MP, Waage JK (1984) Host-parasitoid population interactions. Ann Rev Entomol 29:89–114
Hassell MP, Waage JK, May RM (1983) Variable parasitoid sex ratios and their effect on host-parasitoid dynamics. J Anim Ecol 52:889–904
Horn DJ (1987) Vegetational background and parasitism of larval diamond-back moths on collards. Ent Exp Appl 43:300–303
Kfir R, Luck RF (1979) Effects of constant and variable temperature extremes on sex ratio and progeny production by Aphytis melinus and A. lingnanensis (Hymenoptera: Aphelinidae). Ecol Entomol 4:335–344
King BH (1989) Host-size-dependent sex ratios among parasitoid wasps: does host growth matter? Oecologia 78:420–426
Letourneau DK, Fox LR (1989) Effects of nitrogen and parasitism of lepidopterous larvae on cabbage butterflies. Oecologia 80:211–214
Luck RF, Podoler H (1985) Competitive exclusion of Aphytis lingnanensis by A. melinus: potential role of host size. Ecology 66:904–913
May RM, Anderson RM (1978) Regulation and stability of hostparasite interactions. II. Destabilizing processes. J Anim Ecol 47:249–267
Murdoch WW (1979) Predation and the dynamics of prey populations. Fortschr Zool 25:295–310
Murdoch WW, Stewart-Oaten A (1989) Aggregation by parasitoids and predators: effects on equilibrium and stability. Am Nat 134:288–310
Murdoch WW, Reeve JD, Huffaker CB, Kennett CE (1984) Biological control of scale insects and ecological theory. Am Nat 123:377–392
Murdoch WW, Chesson J, Chesson P (1985) Biological control in theory and practice. Am Nat 125:344–366
Nunney L, Luck RF (1988) Factors influencing the optimum sex ratio in a structured population. Theor Pop Bio 33:1–30
Orion (1986) Model 93–07. Nitrate Electrode Instruction Manual. Orion Research, Inc.
Porter K (1984) Sunshine, sex ratio and behaviour of Euphydryas aurinia larvae. In: Vane-Wright RI, Ackery PR (eds), The Biology of Butterflies. Academic Press, Orlando FL, pp 309–311
Reeve JD, Murdoch WW (1985) Aggregation by parasitoids in the successful control of the California red scale: a test of theory. J Anim Ecol 54:797–816
SAS (1988) SAS/STAT Guide for Personal Computers. Version 6 edition
Sokal RR, Rohlf FJ (1981) Biometry. 2nd edition, Freeman & Co. San Francisco CA
Southwood TR, Comins HN (1976) A synoptic population model. J Anim Ecol 45:949–965
Strong DR (1984) Density-vague ecology and liberal population regulation in insects. In: Price PW, Slobodchikoff CN, Gaud WS (eds), A New Ecology: Novel Approaches to Interactive Systems. Wiley, New York, pp 313–327
Strong DR (1986) Density vagueness: abiding the variance in the demography of real populations. In: Diamond J, Case TJ (eds), Community Biology. Harper & Row, New York, pp 257–268
Vinson SB (1981) Habitat location. In: Nordlund DA, Jones RL, Lewis WJ (eds) Semiochemicals: Their Role in Pest Control, Wiley, New York, pp 51–77
Vinson SB, Iwantsch GF (1980) Host suitability for insect parasitoids. Ann Rev Entomol 25:397–419
Waage JK (1982a) Sib-mating and sex ratio strategies in scelionid wasps. Ecol Entomol 7:103–112
Waage JK (1982b) Sex ratio and population dynamics of natural enemies — some possible interactions. Ann Appl Biol 101:159–164
Waage JK (1983) Aggregation in field parasitoid populations: foraging time allocation by a population of Diadegma (Hymenoptera, Ichneumonidae). Ecol Entomol 8:447–453
Waage JK (1986) Family planning in parasitoids: adaptive patterns of progeny and sex allocation. In: Waage J, Greathead D (eds), Insect Parasitoids. Academic Press, Orlando, FL, pp 63–95
Walde SJ, Murdoch WW (1988) Spatial density dependence in parasitoids. Ann Rev Entomol 33:441–466
Walker TJ (1984) Do populations self-regulate? In: Huffaker B, Rabb RL (eds), Ecological Entomology, Wiley, New York, pp 531–558
Werren JH (1984) A model for sex ratio selection in parasitic wasps: local mate competition and host quality effects. Neth J Zool 34:81–96
Werren JH, Skinner SW, Huger AH (1986) Male-killing bacteria in a parasitic wasp. Science 231:990–992
Williams HJ, Elzen GH, Vinson SB (1988) Parasitoid-host plant interactions emphasizing cotton (Gossypium). In: Barbosa P, Letourneau DK (eds) Novel Aspects of Insect-Plant Interactions. Wiley, NY, pp 171–200
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fox, L.R., Letourneau, D.K., Eisenbach, J. et al. Parasitism rates and sex ratios of a parasitoid wasp: effects of herbivore and plant quality. Oecologia 83, 414–419 (1990). https://doi.org/10.1007/BF00317569
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317569