Summary
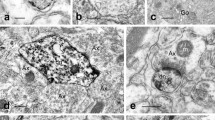
The distribution of cyclic guanosine 3',5' monophosphate (cGMP) producing cells in various organs of the rat were studied immunocytochemically using antibodies raised against formaldehyde-fixed cGMP. Sodium nitroprusside (SNP), a direct activator of guanylate cyclase and vasodilator, was used to enhance cGMP levels. In order to reach all organs optimally, whole body perfusion was performed using a modified Krebs-Ringer buffer at 37° C, aerated with 5% CO2/95% O2, also containing isobutyl methyl xanthine (IBMX); a phosphodiesterase inhibitor. After 15-min pre-perfusion, SNP was added to the perfusate, followed by fast fixation with ice-cold 4% paraformaldehyde-phosphate buffer. After vehicle perfusion, only the retina showed cGMP immunoreactivity in the photoreceptor and ganglion layer, while other organs lacked cGMP immunoreactivity. After 15-min perfusion with SNP (10 μM), enhanced cGMP immunostaining was seen in smooth muscles of the aorta, amacrine-like cells in the retina, glomeruli of the kidney cortex, blood vessels in the dura mater, as well as cells in the pineal and in the median eminence: The results indicate that the distribution and the reactivity of cGMP producing cells, situated outside the blood brain barrier, can be studied by immunocytochemistry after pharmacological manipulations of the intact tissue with a nitrovasodilator using whole body perfusion.
Similar content being viewed by others
References
Ariano MA (1983) Distribution of components of the guanosine 3',5'-phosphate system in rat caudate putamen. Neuroscience 10:707–723
Bartfai T (1980) Cyclic nucleotides in the central nervous system. Curr Top Cell Regul 16:225–269
Chan-Palay V, Palay SL (1979) Immunocytochemical localization of cyclic GMP: light and electron microscopic evidence for involvement of neuroglia. Proc Natl Acad Sci USA 76:1485–1488
Cumming R, Dickison S, Arbuthnott G (1980) Cyclic nucleotide losses during tissue processing for immunocytochemistry. J Histochem Cytochem 28:54–55
De Vente J, Steinbusch HWM, Schipper J (1987a) A new approach to immunocytochemistry of 3',5'-cyclic guanosine monophosphate: preparation, specificity and initial application of a new antiserum against formaldehyde-fixed 3',5'-cyclic guanosine monophosphate. Neuroscience 22:361–373
De Vente J, Garssen J, Tilders FJH, Steinbusch HWM, Schipper J (1987b) Single cell quantitative immunocytochemistry of cyclic GMP in the superior cervical ganglion of the rat. Brain Res 411:120–128
De Vente J, Bol JGJM, Hudson L, Schipper J, Steinbusch HWM (1988) Atrial natriuretic factor-responding and cyclic guanosine monophosphate (cGMP)-producing cells in the rat hippocampus: a combined micropharmacological and immunocytochemical approach. Brain Res 446:387–395
De Vente J, Schipper J, Steinbusch HWM (1989) Formaldehyde fixation of cGMP in distinct cellular pools and their recognition by different cGMP antisera: An immunocytochemical study into the problem of serum specificity. Histochemistry 91:401–412
Dousa TP, Shah SV, Abboud HE (1980) Potential role of cyclic nucleotides in glomerular pathophysiology. In: Hamet P, Sands H (eds) Advances in cyclic nucleotide research, vol 12. Raven Press, New York, pp 285–299
Ferendelli JA, de Vries GW (1983) Cyclic GMP systems in the retina. Fed Proc 42:3103–3106
Gold GH, Nakumura T (1987) Cyclic nucleotide-gated conductances: a new class of ion channels mediates visual and olfactory transduction. Trends Pharmacol Sci 8:312–316
Hancock MB (1982) DAB-Ni substrate for the differential immunoperoxidase staining of nerve fibers and fiber terminals. J Histochem Cytochem 30:578–583
Ignarro LJ, Kadowitz PJ (1985) The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol 25:171–191
Katsuki S, Arnold WP, Mittal CK, Murad F (1977) Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin, and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res 3:23–35
Kaupp UB, Koch KW (1986) Mechanism of photoreception in vertebrate vision. Trends Biol Sci 11:43–47
Lamb TD (1986) Transduction in vertebrate photoreceptors: the role of cyclic GMP and calcium. Trends Neurosci 9:224–228
Ortez RA, Sikes RW, Sperling HG (1980) Immunohistochemical localization of cGMP in goldfish retina. J Histochem Cytochem 28:263–270
Rapoport RM, Murad F (1983) Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J Cyclic Nucleotide Res 9:281–296
Scriabine A (1980) Pharmacology of antihypertensive drugs. Raven Press, New York
Spruill WA, Steiner AL (1979) Cyclic nucleotide and protein kinase immunocytochemistry. Adv Cyclic Nucleotide Res 10:169–186
Steinbusch HWM, De Vente J, Wouterlood FG, Berkenbosch F, Bol JGJM (1988) Immunohistochemical localization of monoamines and cyclic nucleotides. Their application in quantitative immunofluorescence studies and tracing monoaminergic neuronal connections. Acta Histochem J (Suppl) 35:85–106
Steiner AL, Parker CW, Kipnis DM (1972) Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem 247:1106–1113
Steiner AL, Ong S, Wedner HJ (1976) Cyclic nucleotide immunocytochemistry. Adv Cyclic Nucleotide Res 7:115–155
Waldman SA, Murad F (1987) Cyclic GMP synthesis and function. Pharmacol Rev 39:163–196
Wedner HJ, Hoffer BJ, Battenberg E, Steiner AL, Parker CW, Bloom FE (1972) A method for detecting intracellular cyclic adenosine monophosphate by immunofluorescence. J Histochem Cytochem 20:293–299
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berkelmans, H.S., Schipper, J., Hudson, L. et al. cGMP immunocytochemistry in aorta, kidney, retina and brain tissues of the rat after perfusion with nitroprusside. Histochemistry 93, 143–148 (1989). https://doi.org/10.1007/BF00315967
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315967