Summary
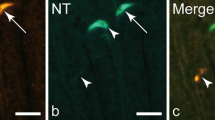
Cathepsins B and H are representative cysteine proteinases localized to lysosomes of a variety of mammalian cells. Previous studies indicated the presence of these enzymes also in secretory granules of endocrine cells. Therefore, the human endocrine pancreas and human insulinomas were investigated by light microscopical immunohistochemistry on serial semithin plastic sections immunostained sequentially for cathepsins B or H and pancreatic hormones. Out of the four established endocrine cell types, insulin (B-) and glucagon (A-) cells showed immunoreactivities for these cathepsins. Cathepsin B immunoreactivities showed a dot-like appearance in A- and B-cells and in insulinoma cells. Immunoreactivities for cathepsin H additionally were found in cell parts containing secretory granules of B-cells and insulinoma cells. By single and double immunoelectron microscopy the dot-like immunoreactivities for cathepsin B were identified as immunoreactive lysosomes of A- and B-cells and insulinoma cells. In addition, some of the secretory granules of A- and B-cells showed cathepsin B immunoreactivities. Cathepsin H immunoreactivities showed an other pattern: they were found regularly in the secretory granules of A- and B-cells and insulinoma cells, and in lysosomes of A-cells. These findings suggest that cathepsins B and H in lysosomes of A- and/or B-cells are involved in the degradation of lysosomal constituents. In secretory granules of these cells, these cystine proteinases may participate in the processing of the corresponding hormones from their precursor proteins.
Similar content being viewed by others
References
Bendayan M (1982) Double immunocytochemical labeling applying the protein A-gold technique. J Histochem Cytochem 30:81–85
Chan SJ, Segundo BS, McCormick MB, Steiner DF (1986) Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc Natl Acad Sci USA 83:7721–7725
Davidson HW, Rhodes CJ, Hutton JC (1988) Intragranular calcium and pH control proinsulin cleavage in the pancreatic β cell via two distinct site-specific endopeptidases. Nature 333:93–96
Docherty K, Corroll RJ, Steiner DF (1982) Conversion of proinsulin to insulin: Involvement of a 31,500 molecular weight thiol protease. Proc Natl Acad Sci USA 79:4613–4617
Fawcett DW, Long JA, Jones AL (1969) The ultrastructure of endocrine glands. Recent Prog Horm Res 25:315–380
Fletcher DJ, Quigley JP, Bauer GE, Noe BD (1981) Characterization of proinsulin- and proglucagon-converting activities in isolated islet secretory granules. J Cell Biol 90:312–322
Fuchs R, Machleidt W, Gassen G (1988) Molecular cloning and sequenceing of a cDNA coding for mature human kidney cathepsin H. Hoppe Seylers Z Physiol Chem 369:469–475
Grube D (1980) Immunoreactivities of gastrin (G-) cells. II. Nonspecific binding of immunoglobulins to G-cells by ionic interactions. Histochemistry 66:149–167
Grube D, Bohn R (1983) The microanatomy of human islets of Langerhans, with special reference to somatostatin (D-) cells. Arch Histol Jpn 46:327–353
Grube D, Kusumoto Y (1986) Serial semithin sections in immunohistochemistry: techniques and applications. Arch Histol Jpn 49:391–410
Grube D, Aunis D, Bader F, Cetin Y, Jörns A, Yoshie S (1986) Chromogranin A (CGA) in the gastro-entero-pancreatic (GEP) endocrine system. I. CGA in the mammalian endocrine pancreas. Histochemistry 85:441–452
Hellerström C (1983) The biosynthesis of glucagon. In: Lefèbvre PJ (ed) Handbook of experimental pharmacology, vol 66/I. Springer, Berlin Heidelberg New York, pp 121–138
Hsu S-M, Raine L, Fanger H (1981) Use of avidin-biotin peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody PAP procedures. J Histochem Cytochem 27:577–580
Ii K, Hizawa K, Kominami E, Bando Y, Katunuma N (1985) Different immunolocalizations of cathepsins B, H, and L in the liver. J Histochem Cytochem 33:1173–1175
Katunuma N, Kominami E (1983) Structures and functions of lysosomal thiol proteinases and their endogenous inhibitors. Curr Top Cell Regul 22:71–101
Kirschke H, Langner J, Riemann S, Wiederanders B, Ansorge S, Bohley P (1979) Lysosomal cysteine proteinases. In: Protein degradation in health and disease. Ciba Found Symp 75:15–35
Kominami E, Katunuma N (1982) Immunological studies on cathepsin B and H from rat liver. J Biochem (Tokyo) 91:67–71
Kominami E, Bando Y, Wakamatsu N, Katunuma N (1984) Different tissue distributions of two types of thiol proteinase inhibitors from rat liver and epidermis. J Biochem (Tokyo) 96:1437–1442
Kominami E, Tsukahara T, Bando Y, Katunuma N (1985) Distribution of cathepsin B and H in rat tissues and peripheral blood cells. J Biochem (Tokyo) 98:87–93
MacGregor RR, Hamilton JW, Kent GN, Shofstall RE, Cohn DV (1979) The degradation of proparathormone and parathormone by parathyroid and liver cathepsin B. J Biol Chem: 4428–4433
Mayor HD, Hampton JC, Rosario B (1961) A simple method for removing the resin form epoxy embedded tissue. J Biophys Biochem Cytol 9:909–910
Nakagawa H, Endo Y, Ohtaki S (1981a) A new protease in hog thyroid lysosomes. I. The presence of a leupeptin-sensitive protease in the soluble fraction of thyroid lysosomes. Acta Endocrinol (Copenh) 98:383–389
Nakagawa H, Endo Y, Ohtaki S (1981b) A new protease in hog thyroid lysosomes. II. A partial purification and characterization of a leupeptin-sensitive protease. Acta Endocrinol (Copenh) 98:390–395
Orci L (1985) The insulin factory: a tour of the plant surroundings and a visit to the assembly line. Diabetologia 28:528–546
Orci L, Ravazzola M, Amherdt M, Yanaihara C, Yanaihara N, Halban P, Renold AE, Perrelet A (1984) Insulin, not C-peptide (proinsulin), is present in crinophagic bodies of the pancreatic B-cell. J Cell Biol 98:222–228
Orci L, Ravazzola M, Amherdt M, Madsen O, Perrelet A, Vassalli J-D, Anderson RGW (1986) Conversion of proinsulin to insulin occurs coordinately with acidification of maturating secretory vesicles. J Cell Biol 103:2273–2281
Orci L, Ravazzola M, Storch M-J, Anderson RGW, Vassalli J-D, Perrelet A (1987) Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell 49:865–868
Richardson KC, Jarett L, Finke EH (1969) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35:313–323
Slot JW, Geuze HJ (1985) A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol 38:87–93
Steiner DF, Docherty K, Chan SJ, San Segundo B, Carroll B (1983) Intracellular proteolytic mechanisms in the biosynthesis of hormones and peptide neurotransmitters. In: Koch G, Richter D (eds) Biochemical and clinical aspects of neuropeptides: synthesis, processing, and gene structure. Academic Press, London New York, pp 3–13
Sternberger LA (1986) Immunocytochemistry, 3rd edn. J Wiley, New York
Takahashi S, Murakami K, Miyake Y (1982) Activation of kidney prorenin by kidney cathepsin B isozyme. J Biochem (Tokyo) 91:419–422
Taugner R, Buhrle CP, Nobiling R, Kirschke H (1985) Coexistence of renin and cathepsin B in epithelioid cell secretory granules. Histochemistry 83:103–108
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Uchiyama Y, Watanabe M, Watanabe T, Ishii Y, Matsuba H, Waguri S, Kominami E (1989a) Variations in immunocytochemical localization of cathepsin B and T4 in follicular cells of the rat thyroid gland and plasma TSH concentration over 24 hours. Cell Tissue Res 256:355–360
Uchiyama Y, Watanabe T, Watanabe M, Ishii Y, Matsuba H, Waguri S, Kominami E (1989b) Localization of cathepsins B, H and L in follicular cells of the rat thyroid gland. J Histochem Cytochem 37:691–696
Wallis M, Howell SL, Taylor KW (1985) The biochemistry of the polypeptide hormones. J Wiley, New York
Watanabe M, Watanabe T, Ishii Y, Matsuba H, Kimura S, Fujita T, Kominami E, Katunuma N, Uchiyama Y (1988) Immunocytochemical localization of cathepsins B, H and their endogenous inhibitor, cystatin β in islet endocrine cells of the rat pancreas. J Histochem Cytochem 36:783–791
Watanabe T, Watanabe M, Ishii Y, Matsuba H, Kimura S, Fujita T, Kominami E, Katunuma N, Uchiyama Y (1989) An immunocytochemical study on co-localization of cathepsins B and atrial natriuretic peptides in secretory granules of atrial myoendocrine cells of the rat heart. J Histochem Cytochem 37:347–351
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Im, B., Kominami, E., Grube, D. et al. Immunocytochemical localization of cathepsins B and H in human pancreatic endocrine cells and insulinoma cells. Histochemistry 93, 111–118 (1989). https://doi.org/10.1007/BF00315963
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315963