Summary
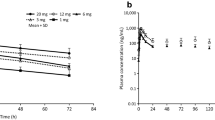
Possible local and systemic adverse effects following administration of salmon (sCT) and human (hCT) calcitonin (CT) have been evaluated in a double-blind, within-subject, comparative trial in 30 young, healthy volunteers. Each subject received 0.25 and 0.5 mg hCT and 100 IU sCT s.c.. Adverse effects and hypocalcaemia were recorded 1, 3 and 6 h after each injection.
Significantly fewer local adverse reactions were observed after hCT (20 or 33%) than after sCT (80%), possibly due to the different vehicles employed (mannitol solution and acetic acid).
The most frequent systemic adverse effects were gastrointestinal (nausea, vomiting), which occurred in 80% after 1 h, independently of the CT — preparation used. Hypocalcaemic changes were generally small and lasted longer after sCT.
It is concluded that the hCT preparations were better tolerated locally than sCT in young, healthy volunteers, and that there were no differences in the systemic side effects or hypocalcaemic activity.
Similar content being viewed by others
References
Stevenson JC et al. (1979) A physiological role for calcitonin: protection of the maternal skeleton. Lancet II: 769
Chambers TJ et al. (1986) The effect of human calcitonin on the cytoplasmic spreading of rat osteoclasts. J Clin Endocrinol Metab 63: 1080–1085
Woodhouse NJY et al. (1970) Some effects of acute and chronic calcitonin administration in man: in Taylor (Ed) Calcitonin 1969. Proceedings of the 2nd International Symposium Heinemann London, 504–513
Chapuy MC, Meunier PJ, Alexandre C (1980) Comparison of the acute effects of human and salmon calcitonins in pagetic patients: Relation to plasma calcitonin levels. Metab Bone Dis Rel Res 2: 93–97
Bataille R, Legendre C, Sany J (1985) Acute effects of salmon calcitonin in multiple myeloma: A valuable method for serial evaluation of osteoclastic lesions and disease activity. A prospective study of 125 patients. J Clin Oncol 3: 229–236
Fournie A, Valverde C, Demblans-Dechans B et al. (1983) Un nouveau test biologique simple d'exploration du squelette: le test a la calcitonine. Rhumatologie 25: 65–69
Milhaud G, Perault AM, Moukhtar MS (1965) Etude de mecanisme d'action hypocalcemiante de la thyreocalcitonine. Comptes Rendues, Academie des Sciences (Paris) 261: 813–816
Rasmussen H, Bordier P (1974) The physiological and cellular basis of metabolic bone disease. Williams and Wilkins, Baltimore, p 292
Marx SJ, Woodard CJ, Auerbach GD (1972) Calcitonin receptors of kidney and bone. Science 178: 999–1001
Ziegler R (1983) Therapeutic use of human calcitonin: General conclusions in Proceedings of the International Workshop on Human Calcitonin of Stresa, April 24th 1982, Ed. A. Caniggia. Ciba-Geigy S. p. A.: 167–175
MacIntyre I, Evans IMA, Woodhouse NJY (1979) Paget's disease. In: De Groot et al. (eds) Endocrinology, Vol 2., Grune and Stratton, New York, pp 891–900
Stevenson JC, Evans JMA (1981) Pharmacology and therapeutic use of calcitonin. Drugs 21: 257–272
Whyte MP et al. (1985): Osteolytic Paget's bone disease in a young man, rapid healing with human calcitonin therapy. Am J Med 78: 326–332
Horowith M et al. (1976): Hereditary bone dysplasia with hyperphosphatasemia: response to synthetic human calcitonin. Clin Endocrinol 5 [Suppl] 341–352
Dunn V et al. (1979) Familial hyperphosphatasemia: diagnosis in early infancy and response to human thyrocalcitonin therapy. AJR 132: 541–545
Ziegler R et al. (1987) Osteogenesis imperfecta and juvenile osteoporosis. Behandlungsversuche mit synthetischem Human-calcitonin. Therapiewoche 37: 1895–1904
Courtillon A et al. (1987) La calcitonine dans le traitement des algodystrophies reflexes recentes des membres inferieurs. Essai controle chez 80 patients en cours de reeducation. Rhumatologie 197: 215–221
Gobelet C (1986): Place de la calcitonine dans le traitement de l'algodystrophie. Schweiz Rundschau Med 73: 7–9
MacIntyre I et al. (1988) Calcitonin for prevention of postmenopausal bone loss. Lancet I: 900–902
Gruber HE, Ivey JL, Baylink DJ, Matthews M, Nelp WB, Sisom K, Chesnut CH III (1984) Long-term calcitonin therapy in postmenopausal osteoporosis. Metabolism 33: 295–303
Binstock ML, Mundy GR (1980) Effect of calcitonin and glucocorticoids in combination on the hypercalcaemia of malignancy. Ann Int Med 93: 269–272
Koelmeyer TD, Stephans EJW (1978) Synthetic human calcitonin in the treatment of hypercalcaemia of metastatic breast cancer: Preliminary report. N Z Med J 87: 434–435
Gennari C, Passeri M, Chierichetti SM, Piolini M (1983) Side-effects of synthetic salmon and human calcitonin. Lancet I: 594–595
Attali G (1985) Etude comparative en triple insu des effects et de la tolerance de la calcitonine sequence humaine versus calcitonine sequence saumon. Gaz Méd 92: 95–98
Langer B, Peytremann A, Rufener C, Jenny M (1971) Effects compares de l'administration d'une chose unique de calcitonine synthetique (de type humain et de type saumon), chez l'homme normal et le sujet atteint de la maladie de Paget ou d'hypercalcemie. Schweiz Med Wochenschr 101: 69–70
Stevenson J (1983) Side-effects of calcitonins. Lancet I: 926
Singer FR, Melvin KEW, Mills BG (1976) Acute effects of calcitonin on osteoclasts in men. Clin Endocrinol 5: 333
Haas HG (1972) Renal effects of calcitonin and parathyroid extract in man. J Clin Invest 50: 2689–2702
Hobitz H (1982) Entwicklung, Chemie und klinische Pharmakologie von vollsynthetischem Humankalzitonin. Wien Klin Wochenschr 94: 67–75
Del Rio A, Rico H, Bordin e, Novoa D (1987) Calcitonin-induced hypocalcaemia as a possible index of osteoclastic activity in patients with chronic renal failure. Nephron 47: 241–245
Sexton P (1987) Localization and characterization of renal calcitonin receptors by in vitro autoradiography. Kidney. Int 32: 862–868
Huwyler R (1979) Plasma kinetics and urinary excretion of exogenous human and salmon calcitonin in man. Am J Phys 236: E15-E19
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wüster, C., Schurr, W., Scharla, S. et al. Superior local tolerability of human versus salmon calcitonin preparations in young healthy volunteers. Eur J Clin Pharmacol 41, 211–215 (1991). https://doi.org/10.1007/BF00315432
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315432