Summary
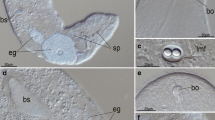
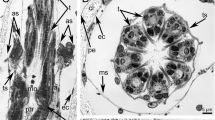
The nervous system of the actinotroch larva of Phoronis muelleri has been investigated with the transmission electron microscope (TEM). Attempts have been made to localize all of the major nerves and to reveal the cytoarchitecture of the apical ganglion. The nervous system is intraepithelial in position and consists of an apical ganglion, located on the epistome, with at least four different cell types, including monopolar sensory cells and mono- or multipolar neuron-like cells. From the anterior part of the apical ganglion three median nerves extend to the edge of the epistome; two of these nerves connect to nerves which follow the edge of the epistome all the way to the junction of the epistome and the mesosome. From the posterior part of the ganglion extend two lateral nerves which continue along the tentacular ring. Each tentacle has three nerves located on the frontal side which connect to the nerve ring along the tentacles. Along the posterior ciliary band is a minor nerve ring. In addition, a nerve net is found on the epistome, mesosome, and metasome, but no longitudinal nerves were observed between the posterior ciliary band and the apical ganglion. All nerve cells were found in the apical ganglion and none was observed along the nerves. Sensory cells (probably mechanoreceptors) are located in two rows on each tentacle; sensory organs such as eyes and statocysts were not observed.
Similar content being viewed by others
Abbreviations
- ac :
-
accessory centricle
- aen :
-
anterior epistome edge nerve
- af :
-
abfrontal cells
- bl :
-
basal lamina
- bl.c :
-
blastocoel coelomocyte
- ci :
-
cilium
- co :
-
collar
- cp :
-
cell process
- cr :
-
ciliary root
- ec 1 :
-
epistome edge cell type 1
- mne :
-
mouth nerve ring
- mo :
-
mouth
- mp :
-
metasomal pouch
- ms :
-
mesosome
- mt :
-
metasome
- mu :
-
muscle
- n :
-
nerve process
- ne :
-
nerve
- np :
-
neuropil
- nu :
-
nucleus
- pc 1 :
-
posterior ciliary band cell type 1
- ec 2 :
-
epistome edge cell type 2
- ec 3 :
-
epistome edge cell type 3
- epi :
-
epidermis
- es :
-
epistome
- ese :
-
epistome edge
- fc :
-
frontal cell
- gc 1 :
-
type 1 ganglion cells
- gc 2 :
-
type 2 ganglion cells
- gc 3 :
-
type 3 ganglion cells
- ge :
-
gut epithelium
- ij :
-
intermediate junction
- laf :
-
lateroabfrontal cell
- lc :
-
lateral cell
- lfc :
-
laterofrontal cell
- lgc :
-
lateral ganglion cell
- me :
-
metacoel epithelium
- lne :
-
longitudinal median epistome nerves
- pc 2 :
-
posterior ciliary band cell type 2
- pc :
-
procoel
- pe :
-
procoel epithelium
- pen :
-
posterior epistome edge nerve
- pr :
-
posterior ciliary band
- p.rec :
-
proximal recess of procoel epithelium
- prne :
-
nerve ring along posterior ciliary band
- sj :
-
septate junction
- sne :
-
secondary nerve along the tentacular ring
- t :
-
tentacle
- tr :
-
tentacular ring
- trne :
-
horseshoe-shaped nerve along the tentacular ring
References
Anderson DT (1973) Embryology and phylogeny in annelids and arthopods. Pergamon Press, Oxford New York Toronto Sydney Braunschweig, pp 1–495
Brandenburger JL et al. (1973) Fine structure of eyespots in tornaria larvae (phylum: Hemichordata). Z Zellforsch 142:89–102
Brooks WK, Cowles RP (1905) Phoronis architecta: Its life history, anatomy, and breeding habits. National Academy of Sciences 10:71–113, plates I–XVII
Bullock TH, Horridge GA (1965) Structure and function in the nervous system of invertebrates, vol 1. Freeman, San Francisco London
Burke RD (1978) The structure of the nervous system of the pluteus larva of Strongylocentrotus purpuratus. Cell Tissue Res 191:233–247
Burke RD (1983a) The structure of the larval nervous system of Pisaster ochraceus (Echinodermata: Asteroidea). J Morphol 178:23–35
Burke RD (1983b) Development of the larval nervous system of the sand dollar, Dendraster excentricus. Cell Tissue Res 229:145–154
Burke RD et al. (1986) Structure of the auricularia larva of Parastichopus californicus. Biol Bull 170:450–460
Burke RD (1987) Development of the nervous system of the pluteus larva of Strongylocentrotus droebachiensis. Cell Tissue Res 248:335–343
Chia F-S, Burke RD (1978) Echinoderm metamorphosis: Fate of larval structures. In: Chia F-S, Rice ME (eds) Settlement and metamorphosis of marine invertebrate larvae. Elsevier/North-Holland, New York, pp 219–234
Chia F-S, Koss R (1979) Fine structural studies of the nervous system and the apical organ in the planula larva of the sea anomone Anthopleura elegantissima. J Morphol 160:275–298
Cobb JLS, Pentreath VW (1977) Anatomical studies of simple invertebrate synapses utilizing stage rotation electron microscopy and densiometry. Tissue and Cell 9:125–135
Cori CI (1939) Phoronidea. Bronn's Kl Ordn Tierreichs 4:1:1
Forneris L (1959) Phoronidea from Brazil. Bolm Inst Oceanogr, S Paulo 10:1–105
Franzén Å, Sensenbaugh T (1983) Fine structure of the apical plate in the larva of the freshwater bryozoan Plumatella fungosa (Pallas) (Bryozoa: Phylactolaemata). Zoomorphology 102:87–98
Gilmour THJ (1978) Ciliation and function of the food-collecting and waste-rejecting organs of lophophorates. Can J Zool 56:2142–2155
Herrmann KV (1976) Untersuchungen über Morphologie, Physiologie und Ökologie der Metamorphose von Phoronis muelleri (Phoronida). Zool Jb Anat 95:354–426
Ikeda I (1901) Observations on the development, structure and metamorphosis of actinotrocha. University of Tokyo, J Coll Sci, Imp 13:507–592
Kalt MR, Tandler B (1971) A study of fixation of early amphibian embryos for electron microscopy. J Ultrastruct Res 36:633–645
Knight-Jones EW (1954) Relations between metachronism and the direction of ciliary beat in Metazoa. Q J Microsc Sci 95:503–521
Kupelwieser H (1905) Untersuchungen über den feineren Bau und die Metamorphose des Cyphonautes. Zoologica (Stuttg) 47:1–50, plates 1–5
Lacalli TC (1981) Structure and development of the apical organ in trochophores of Spirobranchus polycerus, Phyllodoce maculata and Phylodoce mucosa (Polychaeta). Proc R Soc Lond [B] 212:381–402
Lacalli TC (1983) The brain and central nervous system of Müller's larva. Can J Zool 61:39–51
Lacalli TC (1984) Structure and organization of the nervous system in the trochophore larva of Spirobranchus. Phil Trans R Soc London 306:79–135
Lacalli TC, West JE (1985) The nervous system of a pilidium larva: evidence from electron microscope reconstructions. Can J Zool 63:1909–1916
Long JA (1964) The embryology of three species representing three superfamilies of articulate Brachiopoda. University Microfilms International, Ann Arbor, Mich, USA
Masterman AT (1898) On the Diplochorda. Q J Microsc Sci 40:281–366, plates 18–26
Morgan TH (1891) The growth and metamorphosis of tornaria. J Morphol 5:407–458, plates 24–28
Morgan TH (1894) The development of Balanoglossus. J Morphol 9:1–86, plates 1–7
Müller J (1846) Bericht über einige neue Thierformen der Nordsee. Arch Anat Physiol 1846:101–104, plate 5
Nielsen C (1971) Entoproct life-cycles and the entoproct/ectoproct relationship. Ophelia 9:209–341
Reed CG, Cloney RA (1982) The settlement and metamorphosis of the marine bryozoan Bowerbankia gracilis (Ctenostomata: Vesicularioidea). Zoomorphology 101:103–132
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Sensenbaugh T, Franzén Å (1987) Fine structural observations of the apical organ in the larva of Polygordius (Annelida: Polychaeta). Scanning Microscopy 1:181–189
Silen L (1954) On the nervous system of Phoronis. Ark Zool 6:1–40
Strathmann R (1973) Function of lateral cilia in suspension feeding of lophophorates (Brachiopoda, Phoronida, Ectoprocta). Mar Biol 23:129–136
Strathmann R (1982) Comment on Dr. Gilmour's views on feeding by hemichordates and lophophorates. Can J Zool 60:3466–3468
Zimmer RL (1964) Reproductive biology and development of Phoronida. University Microfilms, Ann Arbor, Mich
Zimmer RL (1978) The comparative structure of the preoral hood coelom in Phoronida and the fate of this cavity during and after metamorphosis. In: Chia F-S, Rice ME (eds) Settlement and metamorphosis of marine invertebrate larvae. Elsevier/North-Holland, New York, pp 23–40
Zimmer RL, Woollacott RM (1977) Metamorphosis, ancestrulae, and coloniality in bryozoan life cycles. In: Woollacott RM, Zimmer RL (eds) Biology of bryozoans. Academic Press, New York San Francisco London, pp 91–131
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hay-Schmidt, A. The nervous system of the actinotroch larva of Phoronis muelleri (Phoronida). Zoomorphology 108, 333–351 (1989). https://doi.org/10.1007/BF00312274
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00312274