Summary
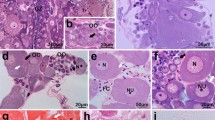
Scypha ciliata is a syconoid sponge. Its oocytes differentiate from choanocytes located near the apopyle of a flagellated chamber, and initially they remain in that location, in a trophic complex with neighbouring choanocytes. When this first growth phase is completed, the oocyte migrates to the periphery of the sponge. There it undergoes a second growth phase, in which it phagocytizes choanocytes and mesenchyme cells.
Fertilization of the mature egg is assisted by a converted choanocyte, the sperm carrier cell. This cell penetrates the oocyte and transfers to it the sperm contained in a carriercell vacuole. No meiotic events have yet been observed.
Cleavage is asynchronous, with holoblastic, approximately equal divisions. After the first cleavage steps the blastomeres often contain multiple nuclei. The single-layered blastoderm of the stomoblastula consists of many micromeres with flagella that project into the blastocoel, a few macromeres and four cruciform cells. There is no development of a follicle epithelium.
The stomoblastula develops into the amphiblastula by inversion; with the assistance of the maternal choanocyte epithelium, the hollow sphere turns inside out, simultaneously moving out of the mesoderm and into the lumen of the adjacent flagellated chamber. In this process, the blastocoel of the stomoblastula is lost. The flagellated cells that form the wall of the amphiblastula now have their flagella extending outward; the amphiblastula also comprises four cruciform cells, macrogranular and agranular cells. The larval cavity of the amphiblastula is a newly formed structure.
Similar content being viewed by others
Abbreviations
- AB :
-
amphiblastula
- AP :
-
apopyle
- BC :
-
blastocoel
- aC :
-
agranular cell
- maC :
-
macrogranular cell
- miC :
-
microgranular cell
- CB :
-
crystalline body
- CC :
-
central cavity
- Ch :
-
choanocyte
- fCh :
-
flat choanocyte
- gCh :
-
granulate choanocyte
- CM :
-
cell membrane
- Co :
-
collar of choanocyte
- CrC :
-
cruciform cell
- DM :
-
dense material
- EM :
-
electron micrograph
- F :
-
flagellum
- FC :
-
flagellated cell
- FCm :
-
flagellated chamber
- FL :
-
free larva
- FV :
-
food vacuole
- IR :
-
interior region
- LC :
-
larval cavity
- M :
-
mesenchyme
- Ma :
-
macromere
- MC :
-
mesenchyme cell
- Mi :
-
micromere
- N :
-
nucleus
- Nu :
-
nucleolus
- O :
-
opening
- OC :
-
oocyte
- P :
-
psudopodium
- PC :
-
pinacocyte
- PhM :
-
phase-contrast micrograph
- Po :
-
pore
- PP :
-
prosopyle
- S :
-
sperm
- SB :
-
stomoblastula
- SC :
-
segmentation cavity
- SCC :
-
sperm-carrier cell
- SV :
-
sperm vacuole
- lT :
-
large trophocyte
- sT :
-
small trophocyte
- V :
-
vacuole
- VC :
-
vesicular cytoplasm
- VM :
-
vacuole membrane
References
Bergquist PR (1978) Sponges. University of California Press, Los Angeles, pp 1–268
Brien P, Meewis H (1938) Contribution a l'étude de l'embryogénèse des spongillidae. Arch Biol 49:177–250
Burton M (1963) A revision of the classification of the calcareous sponges. Clones, London, pp 1–693
Diaz J-P (1979) Variations, différenciations et fonctions des catégories cellulaires de la démosponge d'eaux saumâtres, Suberites massa Nardo, au cours du cycle biologique annuel et dans des conditions expérimentales. Thèse del'Université Scientifique et Technique Languedoc, pp 1–332
Diaz J-P, Connes R (1980) Etude ultrastructurale de la spermatogénèse d'une démosponge. Biol Cell 38:225–230
Diaz J-P, Connes R, Paris J (1973) Origine de lignée germinale chez une démosponge de l'etang de Thau: Suberites massa-Nardo. C R Acad Sci 227:661–664
Diaz J-P, Connes R, Paris J (1975) Etude ultrastructurale de l'ovogénèse d'une démosponge: Suberites massa Nardo. J Microsc 24:105–116
Duboscq O, Tuzet O (1933) Quelques structures des amphiblastules d'éponges calcaires. C R Acad Sci 197:561–563
Duboscq O, Tuzet O (1935a) Sur l'accroissement des ovocytes de Sycon raphanus O.S.: la signification des “dolly-cells”. Arch Zool Exp Gen 77:71–78
Duboscq O, Tuzet O (1935b) Un nouveau stade du développement des éponges calcaires. C R Acad Sci 200:1788–1790
Duboscq O, Tuzet O (1937) L'ovogénèse, la fécondation, et les premiers stades du développement des éponges calcaires. Arch Zool Exp Gen 79:157–316
Duboscq O, Tuzet O (1938) L'origine et l'évolution des cellules en croix des éponges calcaires. Trav Stat Zool Wimereux 13:267–275
Duboscq O, Tuzet O (1941) Sur les cellules en croix des Sycon (Sycon ciliatum Fabr., Sycon coronatum Ellis et Soll., Sycon elegans Bower.) et leur signification. Arch Zool Exp Gen 81:151–163
Duboscq O, Tuzet O (1942) Recherches complémentaires sur l'ovogénèse, la fécondation, et les premiers stades du développement des éponges calcaires. Arch Zool Exp Gen 81:395–466
Duboscq O, Tuzet O (1944) L'ovogénèse, la fécondation et les premiers stades du développement de Sycon elegans Bower. Arch Zool Exp Gen 83:445–459
Fell PE (1974) Porifera. In: Giese AC, Pearse JS (eds) Reproduction of marine invertebrates, vol 1. Academic Press, New York London, pp 51–132
Fell PE (1983) Porifera. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of invertebrates, vol 1. Oogenesis, oviposition and oosorption. Wiley, Chichester, pp 1–29
Gaino E, Burlando B, Buffa P (1986) Contribution to the study of egg development and derivation in Oscarella lobularis (Porifera, Demospongiae). Int J Invertebrate Reprod Dev 9:59–69
Gaino E, Burlando B, Zunino L, Pansini M, Buffa P (1984) Origin of male gametes from choanocytes in Spongia officinalis (Porifera, Demospongiae). Int J Invertebrate Reprod Dev 7:83–93
Gallissian M-F (1980) Etude ultrastructurale de la fécondation chez Grantia compressa. Int J Invertebrate Reprod Dev 2:321–329
Gallissian M-F (1981) Etude ultrastructurale de l'ovogénèse chez quelques éponges calcaires (Porifera, Calcarea). Arch Zool Exp Gen 122:329–340
Gallissian M-F (1983) Etude ultrastructurale du développement embryonnaire chez Grantia compressa F (Porifera, Calcarea). Arch Anat Microsc Morphol Exp 72:59–75
Gatenby J-B (1920a) The germ-cells, fertilization and early development of Grantia (Sycon) compressa. J Lin Soc 34:261–297
Gatenby J-B (1920b) Further notes on the oogenesis and fertilization of Grantia compressa. J Microsc Soc 40:277–282
Gatenby J-B (1927) Further notes on the gametogenesis and fertilization of sponges. Q J Microsc Sci 71:173–188
Gatenby J-B, King S-B (1929) Note on the nutrient membrane of Grantia amphiblastula. J Microsc Soc 49:319–320
Hammer E (1908) Neue Beiträge zur Kenntnis der Histologie und Entwicklung von Sycon raphanus. Arch Biont 2:289–334
Höhr D (1977) Differenzierungsvorgänge in der keimenden Germmula von Ephydatia fluviatilis. W Roux' Arch 182:329–346
Jörgensen M (1910) Beiträge zur Kenntnis der Eibildung, Reifung und Furchung bei Schwämmen (Syconen). Arch Zellforsch 4:163–242
Langenbruch P-F (1981) Zur Enstehung der Gemmulae bei Ephydatia fluviatilis L. (Porifera). Zoomorphology 97:263–284
Langenbruch P-F (1983) Body structure of marine sponges. 1. Arrangement of the flagellated chambers in the canal system of Reniera sp. Mar Biol 75:319–325
Lévi C (1956) Etude des Halisarca de Roscoff. Embryologie et systématique des démosponges. Arch Zool Exp Gen 93:1–181
Lévi C (1963) Gastrulation and larval phylogeny in sponges. In: Dougherty EC (ed) The lower Metazoa. Comparative biology and phylogeny. University of California Press, Berkeley, pp 375–382
Lufty RG (1957) On the placental membrane of calcareous sponges. Cellule 58:239–246
Paulus W, Weissenfels N (1986) The spermatogenesis of Ephydatia fluviatilis (Porifera). Zoomorphology 106:155–162
Saller U, Weissenfels N (1985) The development of Spongilla lacustris from the oocyte to the free larva (Porifera, Spongillidae). Zoomorphology 105:367–374
Sarà M (1974) Sexuality in the Porifera. Boll Zool 41:327–348
Sarà M, Relini Orsi L (1975) Sex differentiation in Sycon (Porifera, Calcispongiae). Pubbl Stan Zool Napoli 39 [Suppl]:307–316
Schulze FE (1875) Über den Bau und die Entwicklung von Sycandra raphanus Haeckel. Z Wiss Zool Abt A 25 [Suppl]:247–280
Schulze FE (1876) Zur Entwicklungsgeschichte von Sycandra. Z Wiss Zool Abt A 27:486–487
Schulze FE (1878) Untersuchungen über den Bau und die Entwicklung der Spongien. Fünfte Mittheilung. Die Metamorphose von Sycandra raphanus. Z Wiss Zool Abt A 31:262–295
Simpson TL (1980) Reproductive processes in sponges: a critical evaluation of current data and views. Int J Invertebrate Reprod Dev 2:251–269
Simpson TL (1984) The cell biology of sponges. Springer Verlag, New York Berlin Heidelberg Tokyo, pp 1–662
Tuzet O (1963) The phylogeny of sponges according to embryological, histological, and serological data, and their affinities with the protozoa and the cnidaria. In: Dougherty EC (ed) The lower Metazoa. Comparative biology and phylogeny. University of California Press, Berkeley, pp 129–148
Weissenfels N (1982) Bau und Funktion des Süßwasserschwammes Ephydatia fluviatilis L. (Porifera). IX. Rasterelektronenmikroskopische Histologie und Cytologie. Zoomorphology 100:75–87
Weissenfels N, Schäfer D (1985) Kombinierte phasenkontrast-und rasterelektronenmikroskopische Histologie. Leitz Mitt Wiss Tech 8:244–246
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Franzen, W. Oogenesis and larval development of Scypha ciliata (Porifera, Calcarea). Zoomorphology 107, 349–357 (1988). https://doi.org/10.1007/BF00312218
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00312218