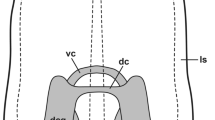
Summary
Pilidium larvae at different developmental stages have been investigated for the occurrence of glyoxylic acid induced fluorescence in catecholamines (CA), and serotonin-like (5-HT) and neuropeptide FMRFamide-like (FMRFamide) immunoreactivity (ir). The distribution of CA, 5-HT-ir and FMRFamide-ir cells and processes was compared with the location of nerve processes as found by transmission electron microscopy (TEM). In the pilidium larvae the marginal and oral nerves contain CA and 5-HT-ir processes and 5-HT-ir unipolar cells. The posterior suboral nerve contain CA and 5-HT-ir processes, whereas in the anterior suboral nerve neither CA nor 5-HT-ir and FMRFamide-ir were observed. The lateral helmet nerve contains FMRFamide-ir processes and unipolar cells. In the epidermis CA and 5-HT-ir multipolar cells were found. The juvenile “worm” that develops inside the pilidium larva was found to contain only 5-HT-ir. A pair of lateral cords extent the whole length of the juvenile and anteriorly they form the anterior ventral cerebral commissure. Also, from the anterior part of the lateral cords projects a pair of circumrhynchodeal processes which dorsally form the dorsal cerebral commissure. A pair of proboscis processes originate from the circumrhynchodeal processes and extend the whole length of the probosics. From the dorsal cerebral commissure cephalic processes project rostrally and ventrally. Only unipolar 5-HT-ir cells were observed, and they were located along the lateral cords into which their processes extend.
Similar content being viewed by others
Abbreviations
- AEC :
-
3-amino-9-ethylcarbazole
- ap :
-
apical plate
- arp :
-
anterior accessory ridge processes
- ason :
-
anterior suboral nerve
- CA :
-
catecholamines
- cd :
-
cephalic discs
- cp :
-
cephalic processes
- crp :
-
circumrhynchodeal processes
- DAB :
-
3,3'-diaminobenzidine
- dc :
-
dorsal cerebral commissure
- epi :
-
epidermis
- es :
-
oesophagus
- fl :
-
fore lobe
- FMRFamide :
-
phe—met—arg—phe—NH2
- Go :
-
goat
- GS :
-
goat serum
- hl :
-
hind lobe
- int :
-
intestine of the juvenile
- lhn :
-
lateral helmet nerve
- lhp :
-
lateral helmet processes
- ll :
-
lateral lobe
- lp :
-
lateral processes of the juvenile
- mcb :
-
marginal ciliary band
- me :
-
mesoderm
- mn :
-
marginal nerve
- moc :
-
monociliary cell
- mp :
-
marginal processes
- mu :
-
muscle
- muc :
-
multiciliary cell
- n 1, n 2, n 3 :
-
division of marginal nerve
- on :
-
oral nerve
- op :
-
oral processes
- pb :
-
proboscis
- pp :
-
proboscis processes
- pson :
-
posterior suboral nerve
- psop :
-
posterior suboral processes
- Ra :
-
rabbit
- sd :
-
stomodeum
- st :
-
stomach
- td :
-
trunk discs
- tr :
-
trunk
- TRITC :
-
tetramethylrhodamine isothiocyanate
- vc :
-
ventral cerebral commissure
- z 1, z 2 :
-
ciliary zones of marginal ciliary band
- 5-HT :
-
serotonin
References
Aiello E (1962) Identification of the cilioexcitatory substance present in the gill of the mussel Mytilus edulis. J Cell Comp Physiol 60:17–21
Aiello E (1974) Control of ciliary activity in metazoa. In: Sleigh MA (ed) Cilia and Flagella, Academic Press, London, pp 353–375
Aiello E, Guideri G (1966) Relationship between 5-hydroxytryptamine and nerve stimulation of ciliary activity. J Pharmacol Exp Ther 154:517–523
Aiello E, Hager E (1986) An opioid mechanism modulates dopaminergic control of ciliary activity in the marine mussel Mytilus edulis. In: Stefano GB (ed) CRC Handbook of Comparative Opioid and Related Neuropeptide Mechanisms, Vol 2. CRC Press, Inc. Boca Raton, Florida, pp 233–241
Bisgrove BW, Burke RD (1986) Development of serotonergic neurons in the embryos of the sea urchin, Strongylocentrotus purpuratus. Dev Growth Differ 28:569–574
Bisgrove BW, Burke RD (1987) Development of the nervous system of the pluteus larva of Strongylocentrotus droebachiensis. Cell Tissue Res 248:335–343
Bullock TH, Horridge GA (1965) Structure and function in the nervous system of invertebrates. Vol 1. W.H. Freeman and Company, San Francisco London
Bürger O (1897–1907) Nemertini (Schnurwürmer). Bronn's Kl Ordn Tierreichs 4:1:suppl
Burke RD (1983) Structure of the larval nervous-system of Pisaster ochraceus (Echinodermata: Asteroidea). J Morphol 1787:23–35
Burke RD, Brand DG, Bisgrove BW (1986) Structure of the nervous system of the auricularia larva of Parastichopus californicus. Biol Bull 170:450–460
Cantell C-E (1966) The devouring of the larval tissues during the metamorphosis of pilidium larvae (Nemertini). Ark Zool (Uppsala) 18:489–493
Cantell C-E (1969) Morphology, development, and biology of the pilidium larvae (Nemertini) from the swedish west coast. Zool Bidr (Uppsala) 38:61–112 plates 1–6
Ferrais JD (1978) Neurosecretion in selected nemertina. Zoomorphologie 91:275–287
Gibson R (1972) Nemerteans. Hutchinson and Co, Ltd, London
Gosselin RE (1961) The cilioexcitatory activity of serotonin. J Cell Comp Physiol 58:17–26
Hay-Schmidt A (1990a) Distribution of catecholamine-containing, serotonin-like and neuropeptide FMRFamide-like immunoreactive neurons and processes in the nervous system of the actinotroch larva of Phoronis muelleri (Phoronida). Cell Tissue Res 259:105–118
Hay-Schmidt A (1990b) Catecholamine-containing, serotonin-like and FMRFamide-like immunoreactive neurons and processes in the nervous system of the early actinotroch larva of Phoronis vancouverensis (Phoronida): Distribution and Development. Can J Zool (in press)
Hyman LB (1951) The invertebrates, vol 2: Platyhelminthes and Rhynchocoela. McGraw-Hill Book Company, Inc, New York Toronto London
Iwata F (1958) On the development of the nemertean Micura akkeshiensis. Embryologia 4:103–131
Jennings JB, Davenport IRB, Varndell IM (1987) FMRFamide-like immunoreactivity in turbellarians and nemerteans — evidence for a novel neurovascular coordinating system in nemerteans. Comp Biochem Physiol 86C:425–430
Kalt MR, Tandler B (1971) A study of fixation of early amphibian embryos for electron microscopy. J Ultrastruct Res 36:633–645
Lacalli TC, West JC (1985) The nervous system of a pilidium larva: evidence from electron microscope reconstructions. Can J Zool 63:1909–1916
Malanga CJ, Poll KA (1979) Effects of the cilioexcitatory neurohumors dopamine and 5-hydroxytryptamine on cyclic AMP levels in the gill of the mussel Mytilus edulis. Life Sci 25:365–374
Mizukawa K, Otsuka N, Hattori T (1986) Serotonin-containing nerve fibers in the rat spinal cord: Electron microscopic immunohistochemistry. Acta Med Okayama 40:1–10
Müller J (1847) Fortsetzung des Berichts über einige neue Thierformen der Nordsee. Arch Anat Physiol 1847:159–160
Murakami A (1983) Control of ciliary beat frequency in Mytilus. J Submicrosc Cytol 15:313–316
Murakami A (1987a) Control of ciliary beat frequency in the gill of Mytilus — I. Activation of the lateral cilia by cyclic AMP. Comp Biochem Physiol 86C:273–279
Murakami A (1987b) Control of ciliary beat frequency in the gill of Mytilus — II. Effects of saponin and Brij-58 on the lateral cilia. Comp Biochem Physiol 86C:281–287
Nakajima Y (1987) Localization of catecholaminergic nerves in larval echinoderms. Zool Sci 4:293–299
Nakajima Y (1988) Serotonergic nerve cells of starfish larvae. In: Burke RD, Mladenov PV, Lambert P, Parsley RL (eds) Echinoderm biology. A.A. Balkema, Rotterdam, Brookfield, pp 235–239
Nielsen C (1985) Animal phylogeny in the light of the trochae theory. Biol J Linn Soc 25:243–299
Nielsen C (1987) Structure and function of metazoan ciliary bands and their phylogenetic significance. Acta Zool (Stockholm) 68:205–262
Paparo AA (1986a) Average ciliary beat in the oyster: Response to photoperiod, pentylenetetrazole, salyrgan, serotonin and dopamine. Mar Behav Physiol 12:149–159
Paparo AA (1986b) Neuroregulatory activities and potassium enhancement of lateral ctenidal beating in Crassostrea virginacea. Comp Biochem Physiol 84A:585–588
Rémy C, Brossard D (1986) Immunohistological detection of metenkephalin-like neuropeptide in the brains of nemerteans (triploblastic acoelomate invertebrates). In: Stefano GB (ed) CRC Handbook of comparative opioid and related neuropeptide mechanisms, Vol 1. CRC Press, Inc. Boca Ratoon, Florida, pp 155–163
Reuter M (1987) Immunocytochemical demonstration of serotonin and neuropeptides in the nervous system of Gyrodactylus salaris (Monogenea). Acta Zool (Stockholm) 68:187–193
Reuter M, Lehtonen M, Wikgren M (1988) Immunocytochemical evidence for neuroactive substances in flatworms of different taxa — a comparison. Acta Zool (Stockholm) 69:29–37
Reuter M, Wikgren M, Lehtonen M (1986) Immunocytochemical demonstration of 5-HT-like and FMRF-amide-like substances in whole mounts of Microstomum lineare (Turbellaria). Cell Tissue Res 246:7–12
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Salensky W (1886) Bau und Metamorphose des Pilidium. Z Wiss Zool 43:481–511
Salensky W (1912) Über die Morphogenese der Nemertinen. — I. Entwicklungsgeschichte der Nemertine im Inneren des Pilidiums. Mem Acad Imper Sci St. Petersbourg, Ser 8, 30, No. 10:1–74 plates 1–6
Schmidt GA (1937) Bau und Entwicklung der Pilidien von Cerebratulus pantherinus und marginatus. Zool Jahrb Abt Anat 62:423–448
Stefano GB (1982) Comparative aspects of opioid-dopamine interaction. Cell Mol Neurobiol 2:167–178
Torre JC de la, Surgeon JC (1976) A methodological approach to rapid and sensitive monoamine histofluorescence using a modified glyoxylic acid technique: The SPG method. Histochemistry 49:81–93
Turbeville JM (1986) An ultrastructural analysis of coelomogenesis in the hoplonemertine Prosorhochmus americanus and the polychaete Magelona sp. J Morphol 187:51–60
Wikgren CM (1986) The nervous system of early larval stages of the cestode Diphyllobothrium dendriticum. Acta Zool (Stockholm) 67:155–163
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hay-Schmidt, A. Catecholamine-containing, serotonin-like and neuropeptide FMRFamide-like immunoreactive cells and processes in the nervous system of the pilidium larva (Nemertini). Zoomorphology 109, 231–244 (1990). https://doi.org/10.1007/BF00312190
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00312190