Summary
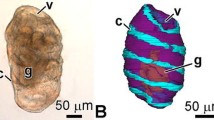
Histological and ultrastructural observations of the digestive tract of eight-armed plutei of Dendraster excentricus are reported. The esophagus is divided into two regions. The uppermost is a narrow tube comprised of ciliated cells that assist in transporting food to the more bulbous lower esophagus where food particles are formed into a bolus prior to entering the stomach. The esophagus is surrounded by a network of smooth muscle fibers that are predominantly oriented circumferentially in the upper esophagus, and longitudinally in the lower esophagus. The musculature of the upper esophagus produces peristaltic contractions, whereas contractions of the muscle of the lower esophagus open the cardiac sphincter and force food from the lower esophagus into the stomach. Axons are associated with the ciliated cells and the muscles of the upper esophagus. The cardiac sphincter consists of a ring of myoepithelium, with cross-striated myofibrils oriented around the bases of the cells. The gastric epithelium is comprised of two cell types. Type I cells, which predominate, absorb and store nutrients, and may be the source of secreted digestive enzymes. Type II cells apparently phagocytize and intracellularly digest whole algal cells. The intestine is comprised of relatively unspecialized cells and probably functions primarily as a conductive tube for the elimination of undigested materials.
Similar content being viewed by others
References
Bacon RL, Julier S (1963) Observations of the fine structure of echinoderm larvae. Anat Rec 145:203
Burke RD (1978) The structure of the nervous system of the pluteus larva of Strongylocentrotus purpuratus. Cell Tissue Res 191:233–247
Burke RD (1980a) Podial sensory receptors and the induction of metamorphosis in echinoids. J Exp Mar Biol Ecol 47:223–234
Burke RD (1980b) Development of pedicellariae in the pluteus larva of Lytechinus pictus (Echinodermata: Echinoidea). Can J Zool 58:1674–1682
Bury H (1896) The metamorphosis of echinoderms. Q J Microscop Sci 38:45–135
Cameron RA, Hinegardner RT (1978) Early events in sea urchin metamorphosis, description and analysis. J Morphol 157:21–32
Chia FS, Burke RD (1978) Echinoderm metamorphosis: fate of larval structures. In: Chia FS, Rice ME (eds) Settlement and metamorphosis of marine invertebrate larvae, Elsevier-North Holland, New York, pp 219–234
Cloney RA, Florey E (1968) Ultrastructure of cephalopod chromatophore organs. Z Zellforsch Mikrosk Anat 89:250–280
Cobb JLS (1968) The fine structure of the pedicellariae of Echinus esculentus I. The innervation of the muscles. J R Microsc Soc 88:211–221
Cobb JLS, Laverack MS (1966) The lantern of Echinus esculentus (L.) III. The fine structure of the lantern retractor muscle and its innervation. Proc R Soc London B164:651–658
Coleman R (1969) Ultrastructure of the tube foot wall of a regular echinoid, Diadema antillarium Philippi. Z Zellforsch Mikrosk Anat 96:162–172
Escholtz F (1829) Zoologischer Atlas Kotzebue Reise, Berl. 1829–1833 III pp 1–261
Florey E, Cahill MA (1977) Ultrastructure of sea urchin tube feet. Evidence for connective tissue involvement in motor control. Cell Tissue Res 177:195–214
Green CR, Bergquist PR, Bullivant S (1979) An anastomosing septate junction in endothelial cells of the phylum echinodermata. J Ultrastruct Res 67:72–80
Gustafson T, Lundgren B, Truefeldt R (1972a) Serotonin and contractile activity in the echinopluteus. A study of the cellular basis of larval behavior. Exp Cell Res 72:115–139
Gustafson T, Ryberg E, Treufeldt R (1972b) Acetylcholine and contractile activity in the echinopluteus. A study of the cellular basis of larval behavior. Acta Embryol Exp 2:199–223
Hinegardner RT (1969) Growth and development of the laboratory cultured sea urchin. Biol Bull 137:465–475
Hyman LH (1955) The invertebrates: Echinodermata. Vol. VI. McGraw Hill Company, New York, pp 1–763
Immers J, Lundgren B (1972) Aspects of differentiation and function of cilia and adjacent structures of the sea urchin larva, Acta Embryol Exp 2:177–197
Kawaguti S (1964) Electron microscopic structure of the podial wall of an echinoid, with special reference to the nerve plexus and the muscle. Biol J Okayama Univ 10:1–12
Lawrence JM, Lawrence AL, Giese AC (1966) Role of the gut as a nutrient storage organ in the purple sea urchin. Phys Zool 39:281–290
MacBride EW (1903) The development of Echinus esculentus together with some points on the development of E. miliaris and E. acutus. Phil Trans R Soc London B195:285–330
Mortensen T (1938) Contributions to the study of development and larval forms of echinoderms IV. Memoires de l'academie Royale des Sciences et des lettres de Danemark Copenhague Section des Sciences 9me serie t VII: 1–59
Pitelka DR (1974) Basal bodies and root structure. In: Sleigh MA (ed) Cilia and Flagella. Academic Press, New York, pp 437–470
Prosser CL, Mackie GO (1980) Contractions of holothurian muscles. J Comp Physiol 136:103–112
Ryberg E (1977) The nervous system of the early echinopluteus. Cell Tissue Res 179:157–167
Ryberg E, Lundgren B (1975) Secretory cells in the foregut of the echinopluteus. Whilhelm Roux's Archiv 177:255–262
Strathmann M (1968) Methods in Development Series. I General Procedures and Echinodermata-Echinoidea. Friday Harbor Laboratories, Friday Harbor
Strathmann RR (1971) The behaviour of planktotrophic echinoderm larvae: mechanisms, regulation, and rates of supension feeding. J Exp Mar Biol Ecol 6:109–160
Strathmann RR (1974) Introduction to function and adaptation in echinoderm larvae. Thal Jugos 10:321–339
Strathmann RR (1975) Larval feeding in echinoderms. Am Zool 15:717–731
Vacquier VD (1971a) The appearance of β-1, 3-glucanohydrase activity during the differentiation of the gut of the sand dollar pluteus. Dev Biol 26:1–10
Vacquier VD (1971b) The effects of glucose and lithium chloride on the appearance of β-1, 3-glucanohydrase activity in sand dollar plutei. Dev Biol 26:11–16
Vacquier VD, Korn LJ, Epel D (1971) The appearance of α-amylase activity during the differentiation of sand dollar plutei. Dev Biol 26:393–399
von Ubisch L (1913) Die Entwicklung von Strongylocentrotus lividus. Z Wiss Zool 106:409–448
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burke, R.D. Structure of the digestive tract of the pluteus larva of Dendraster excentricus (Echinodermata: Echinoida). Zoomorphology 98, 209–225 (1981). https://doi.org/10.1007/BF00312050
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00312050