Summary
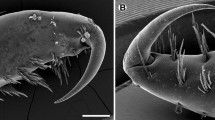
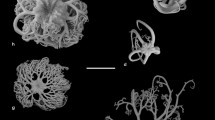
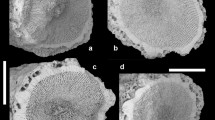
Histological and ultrastructural techniques have been used to describe the functional morphology of clypeasteroid miliary spines, with special reference to their supposed mucus-secreting role. Mucus cells were not found in the miliary spines of any members of the Arachnoididae, Fibulariidae, Laganidae, Echinarachniidae, Dendrasteridae, Astriclypeidae, or Mellitidae examined in this study. Only members of the Clypeasteridae have mucus-secreting cells in these spines. Characteristics of the skeleton, ultrastructure of the nervous system, and histology of the musculature and epithelia of the base, shaft and tip are also discussed. Miliary spines have two bands of cilia running along the entire length of opposite sides of the shaft. The geometric packing of cilium-bearing cells in these bands is described for the first time, as is the remarkable form of the sacs found at the tips of dendrasterid, astriclypeid, and mellitid miliary spines. These sacs are definitely not “mucous sacs”, as previously described, but are balloons of single-celled epithelium internally tethered to the skeletal tip by copious quantities of collagenous connective tissue. Miliary spines prevent obstruction of aboral nutritive and ventilatory ciliary currents caused by substrate particles falling to the test surface during burrowing. They do this in two ways: (1) they help generate ciliary currents that sweep finer material off the test, and (2) they contribute to the formation of a spine canopy that mechanically blocks larger particles from falling between the spines. Members of the Clypeasteridae secrete an interspine mucous tent that traps potentially clogging material. The miliary spine sacs of sand dollars are deformable space-fillers that plug holes between primary spines in the aboral canopy, even as the spines rock on their tubercles to push sand backwards over the test. Allometry of spines from Echinarachnius parma suggests that aboral military spines and club-shaped spines exhibit co-ordinated growth that maintains the aboral canopy throughout post-metamorphic ontogeny, and that aboral spins have an overall lower growth rate than spines on the oral surface.
Similar content being viewed by others
References
Burkhardt A, Hansmann W, Märkel K, Niemann H-J (1983) Mechanical design in spines of diadematoid echinoids (Echinodermata, Echinoidea). Zoomorphology 102:189–203
Ellers O, Telford M (1984) Collection of food by oral surface podia in the sand dollar, Echinarachnius parma (Lamarck). Biol Bull 166:574–582
Florey E, Cahill MA (1977) Ultrastructure of sea urchin tube feet. Evidence for connective tissue involvement in motor control. Cell Tissue Res 177:195–214
Ghiold J (1979) Spine morphology and its significance in feeding and burrowing in the sand dollar, Mellita quinquiesperforata (Echinodermata: Echinoidea). Bull Mar Sci 29:481–490
Ghiold J (1982) Observations on the clypeasteroid Echinocyamus pusillus (O.F. Müller). J Exp Mar Biol Ecol 61:57–74
Ghiold J (1983) The role of external appendages in the distribution and life habits of the sand dollar Echinarachnius parma (Echinodermata: Echinoidea). J Zool Lond 200:405–419
Goodbody I (1960) The feeding mechanism in the sand dollar Mellita quinquiesperforata (Leske). Biol Bull 119:80–86
Hidaka M, Takahashi K (1983) Fine structure and mechanical properties of the catch apparatus of the sea-urchin spine, a collagenous connective tissue with muscle-like holding capacity. J Exp Biol 103:1–14
Holland ND, Nealson KH (1978) The fine structure of the echinoderm cuticle and the subcuticular bacteria of echinoderms. Acta Zool (Stockholm) 59:169–185
Humason GL (1962) Animal tissue techniques. WH Freeman, San Francisco, 569 p
Kawaguti S, Kamishima Y (1965) Electron microscopy on the spine muscle of the echinoid. Biol J Okayama Univ 11:31–40
MacGinitie GE, MacGinitie N (1949) Natural history of marine animals. McGraw-Hill, New York, 473 p
Märkel K, Röser U (1983) The spine tissues in the echinoid Eucidaris tribuloides. Zoomorphology 103:25–31
Mooi R (1986a) Non-respiratory podia of clypeasteroids (Echinodermata, Echinoidea): I. Functional Anatomy. Zoomorphology 106:21–30
Mooi R (1986b) Non-respiratory podia of clypeasteroids (Echinodermata, Echinoidea): II. Diversity. Zoomorphology 106:75–90
Mooi R, Telford M (1982) The feeding mechanism of the sand dollar Echinarachnius parma (Lamarck). Proc Int Echinoderms Conference, Tampa Bay (1981), pp 51–56
Mortensen T (1948) A monograph of the Echinoidea. IV. 2. Clypeastroida. Reitzel, Copenhagen, 471 p
Parker GH, van Alstyne MA (1932) Locomotor organs of Echinarachnius parma. Biol Bull 62:195–200
Seilacher A (1979) Constructional morphology of sand dollars. Paleobiology 5:191–221
Smith AB (1980) The structure and arrangement of echinoid tubercles. Philos Trans R Soc Lond [Biol] 289:1–54
Sokal RR, Rohlf FJ (1981) Biometry. Freeman and Co., San Francisco, 859 p
Stephens GC, Volk MJ, Wright SH, Backlund PS (1978) Transepidermal accumulation of naturally occurring amino acids in the sand dollar, Dendraster excentricus. Biol Bull 154:335–347
Telford M, Harold AS, Mooi R (1983) Feeding structures, behavior, and mcirohabitat of Echinocyamus pusillus (Echinoidea: Clypeasteroida). Biol Bull 165:745–757
Telford M, Mooi R, Ellers O (1985) A new model of deposit feeding in the sand dollar, Mellita quinquiesperforata (Leske) The sieve hypothesis challenged. Biol Bull 169:431–448
Weber W, Grosmann M (1977) Ultrastructure of the basiepithelial nerve plexus of the sea urchin, Centrostephanus longispinus. Cell Tissue Res 175:551–562
Weihe SC, Gray IE (1968) Observations on the biology of the sand dollar Mellita quinquiesperforata (Leske). J Elisha Mitchell Sci Soc 84:315–327
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mooi, R. Structure and function of clypeasteroid miliary spines (Echinodermata, Echinoides). Zoomorphology 106, 212–223 (1986). https://doi.org/10.1007/BF00312042
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00312042