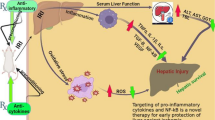
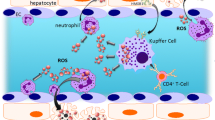
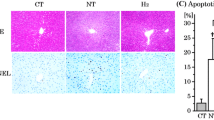
Abstract
It is well known that ischemia causes functional and structural damage to liver cells, and that the status of energy metabolism provides an important means of assessing the functional viability of the ischemic organ. However, the specific sequence leading to ischemic liver cell injury is not yet fully understood; therefore, it is clinically and pathophysiologically important to elucidate the mechanism of cellular injury during hepatic ischemia and subsequent reperfusion. Whereas the conventional view attributes this injury process to the ischemia itself, recent studies have demonstrated that a variable but often substantial proportion of this injury is caused by reactive oxygen metabolites that are generated at the time of reperfusion. This article presents an outline of the mechanism of cellular injury caused during hepatic ischemia and subsequent reperfusion resulting from certain types of surgery, with special reference to the xanthine-xanthine oxidase system and the activation of neutrophils and macrophages.
Similar content being viewed by others
References
McCord JM (1985) Oxygen-derived free radicals in post-ischemic tissue injury. N Engl J Med 312:159–163
Bulkley GB (1987) Free radical-mediated reperfusion injury. A selective review. Br J Cancer 55:66–73
Blankensteijn JD, Terpstra OT (1991) Liver perfusion: The past and the future. Hepatology 13:1235–1250
Clavien PA, Harvey PRC, Slasbery SM (1992) Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation 53:957–978
Chien KR, Abrams J, Serroni A, Martin JT, Farber JL (1978) Accelerated phospholipid degeneration and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem 253:4809–4817
Marubayashi S, Takenaka M, Dohi K, Ezaki H, Kawasaki T (1980) Adenine nucleotide metabolism during hepatic ischemia and subsequent blood reflow periods and its relation to organ viability. Transplantation 30:294–296
Marubayashi S, Dohi K, Ezaki H, Hayashi K, Kawasaki T (1982) Preservation of ischemic rat liver mitochondrial functions and liver viability with CoQ10. Surgery 91:631–637
Marubayashi S, Dohi K, Yamada K, Kawasaki T (1984) Changes in the levels of endogenous coenzyme Q homologs, α-tocopherol, and glutathione in rat liver after hepatic ischemia and reperfusion and the effect of pretreatment with coenzyme Q10. Biochem Biophys Acta 797:1–9
Marubayashi S, Dohi K, Kawasaki T (1986) Role of free radicals in ischemic rat liver cell injury. Prevention of damage by α-tocopherol administration. Surgery 99:184–191
Kawasaki T, Marubayashi S (1986) Liver in shock. In: Barett JM, Nyhus LM (eds) Treatment of shock, 2nd edn. Lea and Febiger, Philadelphia, pp 151–162
Marubayashi S, Dohi K, Sugino K, Kawasaki T (1989) The protective effect of administered α-tocopherol against hepatic damage caused by ischemia reperfusion or endotoxemia. Ann NY Acad Sci 570:208–218
Marubayashi S, Dohi K, Yamada K, Kawasaki T (1991) Role of conversion of xanthine dehydrogenase to oxidase in ischemic rat liver cell injury. Surgery 110:537–593
Marubayashi S, Dohi K, Kawasaki T (1994) Role of free radicals in hepatic reperfusion injury. Ann NY Acad Sci 723:368–370
Mittnacht Jr S, Farber JL (1982) Reversal of ischemic mitochondrial dysfunction. J Biol Chem 256:3199–3206
Sugino K, Dohi K, Yamada K, Kawasaki T (1987) The role of lipid peroxidation in endotoxin-induced hepatic damage and the protective effect of antioxidants. Surgery 101:746–752
Takenaka M, Tatsukawa Y, Dohi K, Ezaki H, Matsukawa K, Kawasaki T (1982) Protective effect of α-tocopherol and coenzyme Q10 on warm ischemic damage of the rat kidney. Transplantation 32:137–141
Yamamoto M, Shima T, Uozumi T, Sogabe T, Yamada K, Kawasaki T (1983) A possible role of lipid peroxidation in cellular damages caused by cerebral ischemia and the protective effect of α-tocopherol administration. Stroke 14:977–982
Sugino K, Dohi K, Yamada K, Kawasaki T (1989) Changes in the level of endogenous antioxidants in the liver of mice with experimental endotoxemia and protective effect of antioxidants. Surgery 105:200–206
Sumimoto K, Inagaki K, Marubayashi S, Kawasaki T, Dohi K (1987) Ischemic damage prevention by coenzyme Q10 treatment of the donor before orthotopic liver transplantation: Biochemical and histologic findings. Surgery 102:821–828
Niki E (1987) Antioxidants in relation to lipid peroxidation. Chem Phys Lipids 44:227–253
MaCay PB, King MM (1980) Vitamin E: Its role as a biologic free radical scavenger and its relationship to the microsomal mixed-function oxidase system. In: Machlin ELJ (ed) Vitamin E. Dekker, New York, pp 289–317
Sonneveld P (1978) Effect of α-tocopherol on the cardiotoxicity of adriamycin in the rat. Cancer Treat Rep 62:1033–1036
Halliwell B (1987) Oxidants and human disease: some new concepts. FASEB J 1:358–364
Folkers K, Liu M, Watanabe T, and Porter TH (1977) Inhibition by adriamycin of the mitochondrial biosynthesis of coenzyme Q10 and implication for the cardiotoxicity of adriamycin in cancer patients. Biochem Biophys Res Commun 77:1536–1542
Flohe L (1979) Glutathione peroxidase: Fact and fiction. In: Ciba Foundation Symposium 65: Oxygen free radicals and tissue damage. Excerpta Medica, Amsterdam, pp 95–122
Tappel AL (1980) Measurement of and protection from in vivo lipid peroxidation. In: Pryor WA (ed) Free radicals in biology, vol 4. Academic, New York, pp 1–47
Arrick BA, Nathan CF, Griffith OW, Cohn ZA (1982) Glutathione depletion sensitizes tumor cells to oxidative cytolysis. J Biol Chem 257:1231–1237
Casini A, Giorli M, Hyland RJ, Serroni A, Gilfor D, Farber JL (1982) Mechanisms of cell injury in the killing of cultured hepatocytes by bromobenzene. J Biol Chem 257:6721–6728
Wendel A, Jaeschke H, Gloger M (1982) Drug-induced lipid peroxidation in mice. Biochem Pharmacol 31:3601–3605
Jaeschke H, Farhood A (1991) Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver in vivo. Am J Physiol G355–362
Jaeschke H (1993) The therapeutic potential of glutathione in hepatic ischemia-reperfusion injury. Transplantation 56:256–257
Witting LA (1980) Vitamin E and lipid antioxidants in free-radical-initiated reactions. In: Pryor WA (ed) Free radicals in biology, vol 4. Academic, New York, pp 295–319
Majewsca MD, Strosznajder J, Lazarewicz J (1978) Effect of ischemic anoxia and barbiturate anesthesia on free radical oxidation of mitochondrial phopholiopids. Brain Res 158:423–434
Adkinson D, Hollwarth ME, Benoit JN, Parks DA, McCord JM, Granger DN (1986) Role of free radicals in ischemia-reperfusion injury to the liver. Acta Physiol Scand 548:101–107
Nordstrom G, Seeman T, Hasselgren PO (1985) Beneficial effect of allopurinol in liver ischemia. Surgery 97:679–684
Hasselgren PO (1987) Prevention and treatment of ischemia of the liver. Surg Gynecol Obstet 164:187–196
Halliwell B, Gutteridge JMC (1986) Iron and free radical reactions: two aspects of antioxidant protection. Trends Biochem Sci 11:372–375
Dohi K, Asahara T, Fukuda Y, Marubayashi S, Yahata T, Hong H (1993) Successful treatment by simultaneous hepatic venoplasty and cavoplasty for Budd-Chiari syndrome with obstruction of retrohepatic inferior vena cava. Surgery 113:574–579
Atalla SL, Toledo-Pereyra LH, Mackenzie GH, Cederna JP (1985) Influence of oxygen-derived free radical scavengers on ischemic livers. Transplantation 40:584–590
Olsen LM, Klintmalm GB, Husberg BS, Nery JR, Whitten CW, Paulsen AW, McClure R (1988) Superoxide dismutase improves organ preservation. Transplant Proc 20:961–964
Komatsu H, Koo A, Ghadishah E, Zeng H, Kulhlenkamp JF, Inoue M, Guth PH, Kaplowitz N (1992) Neutrophil accumulation in ischemic reperfused rat liver. Am J Physiol 262:G669–676
Goode HF, Webster NR, Howdle PD, Leek JP, Lodge JPA, Sadek SA, Walker BE (1994) Reperfusion injury, antioxidants and hemodynamics during orthotopic liver transplantation. Hepatology 19:354–359
Rao PN, Walsh TR, Makowka L, Liu T, Demetris AJ, Rubin RS, Snyder JT, Mischinger HJ, Starzl TE (1990) Inhibition of free radical generation and improved survival by protection of the hepatic microvascular endothelium by targeted erythrocytes in orthotopic liver transplantation. Transplantation 49:1055–1059
Toledo-Pereyra LH, Simmons RL, Nsjarian JS (1975) Protection of the ischemic liver by donor pretreatment before transplantation. Am J Surg 129:513–517
Myakaya G, van Veen H, James J (1984) Ultrastructural changes in rat liver sinusoids during prolonged normothermic and hypothermic ischemia in vitro. Virchows Arch [B] 47:361–373
Hollway CM, Harvey PR, Mullen JB, Strasberg SM (1989) Evidence that cold preservation-induced microcirculatory injury in liver allografts is not mediated by oxygen-free radicals or cell swelling in the rat. Transplantation 48:179–188
Koizumi M, Ohkohochi N, Katoh H, Koyamada N, Fujimori K, Sakurada M, Andoh T, Satomi S, Sasaki T, Taguchi Y, Mori S, Kataoka S, Yamamoto Y (1989) Preservation and reflow damage in liver transplantation in the pig. Transplant Proc 21:1323–1326
McKeown CMB, Edwards V, Philips MJ, Harvey PRC, Petrumka CN, Strasberg SM (1988) Sinusoidal lining cell damage: the critical injury in cold preservation of liver allografts in the rat. Transplantation 46:178–182
Iu S, Harvey PRC, Makowka L, Petrunka CN, IIson RG, Strasberg SM (1987) Markers of allograft viability in the rat: relationship between transplantation viability and liver function in the isolated perfusion liver. Transplantation 44:562–569
Thompson JA, Hess ML (1986) The oxygen free radical system: A fundamental mechanism in the production of myocardial necrosis. Prog Cardiovasc Dis 28:449–462
Fantone JC, Wasrd PA (1985) Polymorphonuclear leukocytemediated cell and tissue injury: Oxygen metabolites and their relations to human disease. Hum Pathol 16:973–978
Kloner RA, Przyklenk K, Whittaker P (1989) Deleterious effects of oxygen radicals in ischemia/reperfusion: Resolved and unresolved issues. Circulation 80:1115–1127
Okuda M, Ikai I, Chance B, Kumar C (1991) Oxygen radical production during ischemia-reperfusion in the isolated perfused rat liver as monitored by luminol enhanced chemiluminescence. Biochem Biophys Res Commun 174:217–221
Brass CA, Nunes F, Nagpal R (1994) Increased oxyradical production during reoxygenation of perfused rat liver. Transplantation 58:1329–1335
Connor H, Gao W, Nukina S, Lemasters JJ, Mason RP, Thurman RG (1992) Evidence that free radicals are involved in graft failure following orthotopic liver transplantation in the rat — an electron paramagnetic resonance spin trapping study. Transplantation 54:199–204
Togashi H, Shinzawa H, Yong H, Takahashi T, Noda H, Okikawa K, Kamada H (1994) Ascorbic acid radical, superoxide, and hydroxyl radical are detected in reperfusion injury of rat liver using electron spin resonance spectroscopy. Arch Biochem Biophys 308:1–7
Granger DN, Rutili G, McCord JM (1981) Role of superoxide radicals in intestinal ischemia. Gastroenterology 81:22–29
Roy RS, McCord JM (1983) Superoxide and ischemia: conversion of xanthine dehydrogenase to xanthine oxidase. In: Greenwaldt RA, Cohen G (eds) Oxyradicals and their scavenger systems, vol 2. Elsevier, New York, pp 145–153
Batelli MG, Molrenzoni E, Stirpe F (1973) Milk xanthine oxidase type D (dehydrogenase) and type O (oxidase). Purification, interconversion and some properties. Biochem J 131:191–198
Della Corte E, Stirpe F (1972) The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J 126:739–745
Parks DA, Bulkley GB, Granger DN (1983) Role of oxygenderived free radicals in digestive tract diseases. Surgery 94:415–422
DeGroot H, Littauer A (1988) Reoxygenation injury in isolated hepatocytes: cell death proceeded conversion of xanthine dehydrogenase to xanthine oxidase. Biochem Biophys Res Commun 155:278–282
Metzger J, Dore SP, Lauterburg BH (1988) Oxidant stress during reperfusion of ischemic liver: no evidence for a role of xanthine oxidase. Hepatology 8:580–584
Yokoyama Y, Beckman JS, Beckman TK, Wheat JK, Cash TG, Freeman BA, Parks DA (1990) Circulating xanthine oxidase: potential mediator of ischemic injury. Am J Physiol 258: G564–570
Toledo-Pereyra LH, Simmons RL, Najarian JS (1974) Effect of allopurinol on the preservation of ischemic kidneys perfused with plasma or plasma substitutes. Ann Surg 180:780–782
Das DK, Engelman RM, Clement R, Otani H, Prasad MR, Rao PS (1987) Role of oxidase inhibitor as free radical scavenger: a novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem Biophys Res Commun 148:314–319
Moorhouse PC, Grootveld M, Halliwell B, Quinlan JG, Gutteridge JMC (1987) Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett 213:23–28
Peterson DA, Kelly B, Gerranrd JM (1986) Allopurinol can act as an electron transfer agent. Is this relevant during reperfusion injury? Biochem Biophys Res Commun 137:76–79
Liu P, McGuire GM, Fisher MA, Farhood A, Smith CW, Jaeschke H (1995) Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock 3:56–62
Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ (1993) Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol 264:G801-G809
Li X, Yamaguchi T, Harada Y (1993) Effect of protease inhibitor on ischemia/reperfusion injury on the rat liver. Transplantation 56:1331–1336
Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D (1973) Liver ischemia and reperfusion injury. Transplantation 55:1265–1272
Klempner MS, Dmarello CA, Gallin JI (1978) Human leukocytic pyrogen induces release of specific granule contents from human neutrophils. J Clin Invest 61:1330–1335
Faymonville ME, Pincemail J, Duchatean J (1991) Myeloperoxidase and elastase as marker of leukocyte activation during cardiopulmonary bypass in humans. J Thorac cardiovasc Surg 102:309–317
Hammerschmidt DE, Harris PD, Wayland H (1981) Complement-induced granulocyte aggregation in vivo. Am J Pathol 102:146–150
Kukreja RC, Hess ML (1993) Oxygen radicals, neutrophilderived oxidants and myocardial reperfusion injury. In: Das KA (ed) Pathophysiology of reperfusion injury. CRC, Boca Raton, pp 221–241
Weiss SJ (1989) Tissue destruction by neutrophils. N Engl J Med 320:365–376
Jaeschke H, Farhood A, Smith CW (1990) Neutrophils contribute to ischemic/reperfusion, injury in rat liver in vivo. FASEB J 4:3355–3359
Andrian UHV, Chambers JD, McEvoy LM, Bargatze RF (1991) Two-step model of leukocyte-endothelial cell interaction: Distinct roles for LECAM-1 and the leukocyte β2 integrins in vivo. Proc Natl Acad Sci USA 88:7538–7542
Albelda SM, Buck CA (1990) Integrins and other adhesion molecules. FASEB J 4:2868–2880
Granger DN, Benoit JN, Suzuki M, Grisham MB (1989) Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol 257:G683-G688
Suzuki M, Inauen W, Kvietys PR, Grisham MB, Maeninger C, Schilling ME, Grange HJ, Granger DN (1989) Superoxide mediates reperfusion-induced leukocyte-endothelial cell interactions. Am J Physiol 257 (Heart Circ Physiol 26): H1740-H1745
Vedder NB, Winn RK, Rice CL, Chi EY, Afrors KE, Hadrian JM (1988) A monoclonal antibody to the adherence-promoting leukocyte glycoprotein CD18 reduces organ injury and improves survival from hemorrhagic shock and resuscitation in rabbits. J Clin Invest 31:939–944
Anderson DC, Springer TA (1987) Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1 and pl50,95 glycoproteins. Annu Rev Med 38:175–194
Smith CW, Marlin SD, Rothlein R, Toman C, Anderson C (1989) Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and trans-endothelial migration of human neutrophils in vitro. J Clin Invest 83:2008–2017
Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ, Smith CW (1993) Functional inactivation of neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology 17:915–923
Jaeschke H, Farhood A, Smith CW (1991) Neutrophil-induced liver cell injury in endotoxin shock is a CD11b/CD18-dependent mechanism. Am J Physiol 261:G1051-G1056
Simpson PJ, Todd RF, Mickelson JK, Fantone JC, Gallagher KP, Lee KA, Tamura T (1990) Sustained limitation of myocardial reperfusion injury by as monoclonal antibody that alters leukocyte function. Circulation 81:226–237
Rosen H, Gordon S (1987) Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J Exp Med 166:1685–1701
Suzuki S, Toledo-Pereyra LH (1994) Monoclonal antibody to intercellular adhesion molecule 1 as an effective protection for liver ischemia and reperfusion injury. Transplant Proc 26:325–327
Toledo-Pereyra LH, Suzuki S (1994) Neutrophils, cytokines, and adhesion molecules in hepatic ischemia and reperfusion injury. Am College Surg 179:758–762
Keely KJ, Williams Jr WW, Colvin RB, Bonventre JV (1994) Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci USA 91:812–816
Smith CW (1992) Transendothelial migration. In: Harlan JM, Liu DY (eds) Adhesion. Its role in inflammatory disease. Freeman, New York, pp 83–115
Ma X, Lefer DJ, Lefer AM, Rothlein R (1992) Coronary endothelial and cardiac protective effects of a monoclonal antibody to intercellular adhesion molecule-1 in myocardial ischemia and reperfusion. Circulation 86:937–946
Clark WM, Madden KP, Rothlein R, Zivin JA (1991) Reduction of central nervous system ischemic injury by monoclonal antibody to intercellular adhesion molecule. J Neurosurg 75:623–627
Oshiro Y, Marubayashi S, Maeda T, Asahara T, Fukuda Y, Yamada K, Koyama S, Ito H, Miyasaka M, Dohi K (1995) Contribution of neutrophils to and effect of monoclonal antibodies of adhesion molecules on ischemia and reperfusion injury in rat liver. Transplant Proc 27:743–744
Maeda T, Marubayashi S, Oshiro Y, Sugino K, Dohi K (1994) Effects of anti-leukocyte-adhesion molecule antibodies, nitric oxide synthase inhibitor, and methyl-prednisolone on endotoxin-shock mice. Europ Surg Res 26:68
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marubayashi, S., Dohi, K. Therapeutic modulation of free radical-mediated reperfusion injury of the liver and its surgical implications. Surg Today 26, 573–580 (1996). https://doi.org/10.1007/BF00311659
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00311659