Summary
It has been shown by many studies that mesangial cell contraction exerts considerable influences on glonerular filtration dynamics. However, experimental findings about the geometrical changes within the glomerular tuft going along with mesangial cell contractions are lacking. This study analyzes the geometry of mesangial cells and their relationship to glomerular capillaries, especially to the glomerular basement membrane (GBM).
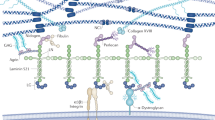
By applying a new staining technique of unosmicated specimens for TEM, the cellular outlines of glomerular cells (mesangial, endothelial and epithelial) and the distribution of extracellular matrices can be more easily studied than in conventionally osmicated specimens. It became obvious that mesangial cells and the GBM are extensively connected with each other, either by direct attachments or indirectly by microfibrils. These connections are especially prominent mesangial angles, i.e. at sites where the GBM deviates from its pericapillary course and covers the mesangium. Thereby, the GBM is not only coupled to the mesangium out—via mesangial cell processes—also to the GBM at the opposing mesangial angle. It seems possible that contraction of mesangial cells can bring the GBM from opposing mesangial angles closer together. Therefore we conclude that the GBM and the contractile mesangial cells together establish a biomechanical unit capable of developing wall tension in glomerular capillaries and of changing the geometry of glomerular capillaries following mesangial contraction or relaxation.
Similar content being viewed by others
References
Andrews PM, Coffey AK (1983) Cytoplasmic contractile elements in glomerular cells. Fed Proc 42:3046–3052
Ausiello DA, Kreisberg JJ, Roy C, Karnovsky MJ (1980) Contraction of cultured rat glomerular mesangial cells after stimulation with angiotensin II and arginine vasopressin. J Clin Invest 65:754–760
Bachmann S, Kriz W, Kuhn C, Franke WW (1983) Differentiation of cell types in the mammalian kidney by immunofluorescence microscopy using antibodies to intermediate filament proteins and desmoplakins. Histochem 77:365–394
Bargmann W, Hehn GV (1971) Über des Nephron der Elasmobranchier. Z Zellforsch 114:1–21
Becker CG (1972) Demonstration of actomyosin in mesangial cells of the renal glomerulus. Am J Pathol 66:97–110
Böck P (1983) Elastic fiber microfibrils: Filaments that anchor the epithelium of the epiglottis. Arch Histol Jpn 46:307–314
Brenner BM, Dworkin LD, Ichikawa I (1986) Glomerular ultrafiltration. In: Brenner BM, Rector FC (eds) The kidney, 3rd edn. Saunders, Berlin. vol 1, pp 124–144
Bretschneider HJ (1980) Myocardial protection. Thorac Cardiovasc Surg 28:295–302
Burton AC (1951) On the physical equilibrium of small blood vessels. Am J Physiol 164:319–329
Carlemalm E, Garavito RM, Villinger W (1982) Resin development for electron microscopy and an analysis of embedding at low temperature. J Microsc 126:123–143
Cleary EG, Gibson MA (1983) Elastin-associated microfibrils and microfibrillar proteins. Int Rev Connect Tissue Res 10: 97–209
Courtoy PJ, Kanwar YS, Hynes RO, Farquhar MG (1980) Fibronectin localization in the rat glomerulus. J Cell Biol 87:691–696
Demmel U, Schewe U, Böck P, Gorgas K (1979) Die Feinstruktur der Muskel-, Sehnen-und Muskel-Epithelverbindungen in der Zunge des Meerschweinchens. Cytobiol 18:460–477
Dworkin LD, Brenner BM (1985) Biophysical basis of glomerular filtration. In: Seldin DW, Giebisch G (eds) The kidney: physiology and pathophysiology, Raven Press, New York, pp 397–426
Essner E, Gordon SR (1984) Demonstration of microfibrils in Bruch's membrane of the eye. Tissue Cell 16:779–788
Farquhar MG (1981) The glomerular basement membrane—a selective macromolecular filter. In: Hay ED (ed) Cell biology of extracellular matrix. Plenum Press, New York, pp 335–378
Farquhar MG, Palade GE (1962) Functional evidence for the existence of a third cell type in the renal glomerulus. Phagocytosis of filtration residues by a distinctive “third” cell. J Cell Biol 13:55–87
Foidart JB, Mahieu P (1986) Glomerular mesangial cell contractility in vitro is controlled by an angiotensin-prostaglandin balance. Mol Cell Endocrinol. 47:163–173
Fullmer HM, Lillie, RD (1958) The oxytalan fiber: a previously underscribed connective tissue fiber. J Histochem Cytochem 6:425–430
Gibson MA, Hughes JL, Fanning JC, Cleary EG (1986) The major antigen of elastin-associated microfibrils is a 31-kDa glycoprotein. J Biol Chem 261:11429–11436
Goldfischer S, Coltoff-Schiller B, Goldfisher M (1985) Microfibrils, elastic anchoring components of the extracellular matrix, are associated with fibronectin in the zonule of zinn and aorta. Tissue Cell 17:441–450
Goldfischer S, Coltoff-Schiller B, Schwarz E, Blumenfeld OO (1983) Ultrastructure and staining properties of aortic microfibrils (oxytalan). J Histochem Cytochem 31:382–390
Goldman RD, Chojnackl B, Yerna MJ (1979) Ultrastructure of microfilament bundles in baby hamster kidney (BHK-21) cells. J Cell Biol 80:759–766
Hanak H, Böck P (1971) Die Feinstruktur der Muskel-Sehnenver-bindung von Skelett-und Herzmuskel. J Ultrastruct Res 36:68–85
Helmchen UE (1980) Die Zahl der Mesangiumzellen in einem normalen Glomerulum der Rattenniere. Eine dreidimensionale elektronenoptische Analyse. Med Diss, Tübingen
Hsu H-C, Churg J (1979) Glomerular microfibrils in renal diseases: A comparative electron microscopic study. Kidney Int 16:497–504
Inoue S, Leblond CP (1986) The microfibrils of connective tissue: I. Ultrastructure. Am J Anat 176:121–138
Inoue S, Leblond CP, Grant DS, Rico P (1986) The microfibrils of connective tissue: II Immunohistochemical detection of the amyloid P component. Am J Anat 176:139–152
Kaissling B, Kriz W (1982) Variability of intercellular spaces between macula densa cells: a transmission electron microscopic study in rabbits and rats. Kidney Int 22 [Suppl 12]:9–17
Kallerhoff M, Blech M, Kehrer G, Kleinert H, Siekmann W, Helmchen U, Bretschneider HJ (1986) Post-ischemic renal function after kidney protection with the HTK-solution of Bretschneider. Urol Res 14:271–277
Kanwar YS (1984) Biology of Disease. Biophysiology of glomerular filtration and proteinuria. Lab Invest 51:7–21
Kreisberg JI (1983) Contractile properties of the glomerular mesangium. Fed Proc 42:3053–3057
Kühn K, Sterzel RB, Stolte H, Reale E (1976) Mesangial cells in different vertebrate kidneys. A thin-section and freeze-fracture study. In: Sterzel RB, Thomson D, Brod J (eds) Contrib Nephrol. Karger, Basel, vol 2, pp 9–16
LaFountain JR, Zobel CR, Thomas HR, Galbreath C (1977) Fixation and Staining of F-Actin and Microfilaments using tannic acid. J Ultrastruct Res 58:78–86
Latta H, Maunsbach AB, Madden SC (1960) The centrolobular region of the renal glomerulus studied by electron microscopy. J Ultrastruct Res 4:455–472
Laurie GW, Leblond CP, Inoue S, Martin GR, Chung (1984) Fine structure of the glomerular basement membrane and immunolocalization of five basement membrane components to the lamina densa (Basal lamina) and its extensions in both glomeruli and tubules of the rat kidney. Am J Anat 169:463–481
Linder E, Miettinen A, Törnroth T (1980) Fibronectin as a marker for the glomerular mesangium in immunohistology of kidney biopsies. Lab Invest 42:70–75
Madri JA, Roll FJ, Furthmayr H, Foidart JM (1980) Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol 86:682–687
Maupin P, Pollard TD (1983) Improved preservation and staining of HeLa cell actin filaments, clathrin-coated membranes, and other cytoplasmic structures by tannic acid-glutaraldehyde-saponin fixation. J Cell Biol 96:51–62
Olesnicky L, Doty SB, Bertani T, Pirani CL (1984) Tubular microfibrils in the glomeruli of membraneous nephropathy. Arch Pathol Lab Med 108:902–905
Pricam C, Humbert F, Perrelet A, Orci L (1974) Gap junctions in mesangial and lacis cells. J Cell Biol 63:349–354
Ross R (1973) The elastic fiber. A review. J Histochem Cytochem 21:199–208
Rostagno A, Frangione B, Pearlstein E, Garcia-Pardo A (1986) Fibronectin binds to amyloid P component. Localization of the binding site to the 31,000 Dalton C-terminal domain. Biochem Biophys Res Commun 140:12–20
Sakai LY, Keene DR, Engvall E (1986) Fibrillin, a new 350-kD glycoprotein is a component of extracellular microfibrils. J Cell Biol 103:2499–2509
Sakai T, Sabanovic S, Hosser H, Kriz W (1986) Heterogeneity of the podocyte membrane in rat kidney as revealed by ethanol dehydration of unosmicated specimens. Cell Tissue Res 246:145–151
Savin VJ (1986) In vitro effects of angiotensin II on glomerular function. Am J Physiol 251:F627-F634
Schnabel PhA, Richter J, Gebhard MM, Pomykaj Th, Preusse CJ, Ulbricht LJ, Bretschneider HJ (1985) Comparison of fixation by immersion and by perfusion after cardioplegia. Verh Anat Ges 79:311–314
Schwartz E, Goldfischer S, Coltoff-Schiller B, Blumenfeld OO (1985) Extracellular matrix microfibrils are composed of core proteins coated with fibronectin. J Histochem Cytochem 33:268–274
Simionescu N, Simionescu M (1976) Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol 70:608–621
Singhal PC, Scharschmidt LA, Gibbons N, Hays RM (1986) Contraction and relaxation of cultured mesangial cells on a silicone rubber surface. Kidney Int 30:862–873
Singer H (1982) Association of fibronectin and vinculin with focal contacts and stress fibers in stationary hamster fibroblasts. J Cell Biol 92:398–408
Skorecki KL, Ballermann BJ, Rennke HG, Brenner BM (1983) Angiotensin II receptor regulation in isolated renal glomeruli. Fed Proc 42:3064–3070
Sraer JD, Sraer J, Ardaillou R, Mimoune D (1974) Evidence for renal glomerular receptors for angiotensin II. Kidney Int 6:241–246
Steinhausen M, Kücherer H, Parekh N, Snoei H (1986) Microcirculation in glomerular network: Implication of hemodynamic factors in effect of angiotensin II on glomerular function. In: Popel Johnson (eds) Microvascular Networks. Experimental and Theoretical Studies. S. Karger, Basel, 1986, pp 134–141
Streeten BW, Licari PA (1983) The zonules and the elastic microfibrillar system in the ciliary body. Invest Ophthalmol Vis Sci 24:667–681
Takagi M, Parmley RT, Yagasaki H, Toda Y (1984) Ultrastructural cytochemistry of oxytalan fibers in the periodontal ligament and microfibrils in the aorta with the periodic acid-thiocarbohydrazide-silver proteinate method. J Oral Pathol 13:671–678
Tanaka T, Fujiwara Y, Orita Y, Sasaki E, Kitamura H, Abe H (1985) The functional characteristics of cultured rat mesangial cells. Jpn Circ J 48:1017–1029
Timpl R (1986) Recent advances in the biochemistry of glomerular basement membrane. Kidney Int 30:293–298
Tsujii T, Naito I, Ukita S, Ono T, Seno S (1984a) The anionic barrier system in the mesonephric renal glomerulus of the arctic lamprey, Entospheus japonicus (Martens) (Cyclostomi). Cell Tissue Res 235:491–496
Tsujii T, Naito I, Ukita S, Ono T, Seno S (1984b) The anionic barrier system in the mesonephric renal glomerulus of the brown hagfish, Paramyxine atami Dean (Cyclostomi). Anat Rec 208:337–347
Vasmant D, Maurice M, Feldmann G (1984) Cytoskeleton ultrastructure of podocytes and glomerular endothelial cells in man and in the rat. Anat Rec 210:17–24
Yoshikawa N, Cameron AH, White RHR, Standring DM (1982) Microfibrils in glomerulopathies of children: an ultrastructural study. J Pathol 136:123–131
Zimmermann KW (1929) Über den Bau des Glomerulus der menschlichen Niere. Z mikr anat Forsch 18:520–552
Author information
Authors and Affiliations
Additional information
Fellow of the Alexander von Humboldt Foundation
Rights and permissions
About this article
Cite this article
Sakai, F., Kriz, W. The structural relationship between mesangial cells and basement membrane of the renal glomerulus. Anat Embryol 176, 373–386 (1987). https://doi.org/10.1007/BF00310191
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00310191