Abstract
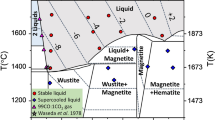
An experimental study using piston-cylinder, Bridgman anvil and diamond anvil cell techniques was undertaken to study the effect of pressure on the composition of Fe x O in equilibrium with Fe. At constant temperature the value of x first increases and then decreases with increasing pressure. The rate of change of x is a function of temperature. We have theoretically calculated the variation of the composition of wüstite with pressure and temperature. The initial increase of x with pressure for P<10 GPa occurs because the partial molar volume of FeO in Fe x O is smaller than the molar volume of Fe, favouring an increase in stoichiometry of Fe x O. To reproduce the experimentally observed decrease in x above 10GPA, the bulk modulus of Fe x O must vary strongly with x for x≳0.96, causing a rapid increase in the partial molar volume of Fe in Fe x O. Continuation of a strong sensitivity of K to x in Fe x O for x≲0.96, however, leads to absurdly low molar volumes of Fe x O at high pressure and no equilibrium between Fe and Fe x O exists. Observations therefore require a reduced sensitivity of K with x for x<0.96, achieved by a negligible variation of K 0 with x for x<0.96, or a strong variation of dK/dP with x, or perhaps both.
Similar content being viewed by others
References
Basinski ZS, Hume-Rothery W, Sutton AL (1955) The lattice expansion of iron. Proc Roy Soc London Ser A 229:459–467
Bassett WA, Ming LC (1972) Disproportionation of Fe2SiO4 to 2FeO+SiO2 at pressures up to 250 kbar and temperatures up to 3,000° C. Phys Earth Planet Inter 6:154–160
Bassett WA, Takahashi T (1965) Silver iodide polymorphs. Am Mineral 50:1576–1594
Birch F (1952) Elasticity and constitution of the earth's interior. J Geophys Res 57:227–286
Bonczar LJ, Graham EK (1982) The pressure and temperature dependence of the elastic properties of polycrystal magnesiowüstite. J Geophys Res 87:1061–1078
Boyd FR, England JL (1960) Apparatus for phase-equilibrium measurements of pressures up to 50 kilobars and temperatures up to 1,750° C. J Geophys Res 65:741–748
Carel C (1967) Recherches expérimentales et theóriques sur le diagramme d'état de la wüstite solide au-dessus de 910° C. Mem Sci Rev Metall 64:821–836
Clendenen RL, Drickamer HG (1966) Lattice parameters of nine oxides and sulfides as a function of pressure. J Chem Phys 44:4223–4228
Darken LS, Gurry RW (1945) The system iron-oxygen. I. The wüstite field and related equilibria. J Am Chem Soc 67:1398–1412
Darken LS, Gurry RW (1953) Physical chemistry of metals. McGraw-Hill, New York
Davies MW, Richardson FD (1959) The non-stoichiometry of manganous oxide. Trans Faraday Soc 55:604–610
Dever DJ (1972) Temperature dependence of the elastic constants in α-iron single crystals: Relationship to spin order and diffusion anomalies. J Appl Phys 43:3293–3301
Fender BEF, Riley FD (1969) Thermodynamic properties of Fe1-x O transitions in the single phase region. J Phys Chem Solids 30:793–798
Foster PK, Welch AJE (1956) Metal-oxide solid solutions. I. Lattice-constant and phase relationships in ferrous oxide (wüstite) and in solid solutions of ferrous oxide and manganous oxide. Trans Faraday Soc 52:1626–1635
Giddings RA, Gordon RS (1973) Review of oxygen activities and phase boundaries in wüstite as determined by electromotive-force and gravimetric methods. J Am Ceram Soc 56:111–116
Green TH, Ringwood AE, Major A (1966) Friction effects and pressure calibration in a piston-cylinder apparatus at high pressure and temperature. J Geophys Res 71:3589–3594
Greenwood NN, Howe AT (1972a) Mössbauer studies of Fe1-x O. Part 1. The defect structure of quenched samples. J Chem Soc Dalton Trans 110–116
Greenwood NN, Howe AT (1972b) Mössbauer studies of Fe1-x O Part II. Disproportionation between 300 K and 700 K. J Chem Soc Dalton Trans 116–121
Guinan MW, Beshers DN (1968) Pressure derivatives of the elastic constants of α-iron to 10 kilobars. J Phys Chem Solids 29:541–549
Hazen RM (1981) Systematic variation of bulk modulus of wüstite with stoichiometry. Ann Rep Geophys Lab 80:277–280
Hentschel B (1970) Stoichiometric FeO as metastable intermediate of the decomposition of wüstite at 225° C. Z Naturforsch 25:1996–1997
Hoffman A (1959) Der Zerfallsmechanismus des Wüstits Fe1-x O unterhalb 570° C. Z Electrochem 33:207–213
Jackson I, Liebermann RC, Ringwood AE (1978) The elastic properties of (Mg x Fe1-x O) solid solutions. Phys Chem Minerals 3:11–31
Jette ER, Foote F (1933) An X-ray study of the wüstite (FeO) solid solutions. J Chem Phys 1:29–36
Katsura T, Iwasaki B, Kimura S, Akimoto S (1967) High-pressure synthesis of the stoichiometric compound FeO. J Chem Phys 47:4559–4560
Kurepin VA (1975) Component activities, thermodynamic characteristics of reactions and phase equilibria in the Fe- O system at high temperatures and pressures. Geochem Int 114–121
Levin RL, Wagner JB (1966) Lattice-parameter measurements of undoped and chromium-doped wüstite. Trans Am Inst Min Metall Pet Eng 236:516–519
Liu LG (1976) The high-pressure phases of FeSiO3 with implications for Fe2SiO4 and FeO. Earth Planet Sci Lett 33:101–106
MacGillavry CH, Rieck GD (eds) (1968) International tables for X-ray crystallography, vol III: Physical and chemical tables. Inter Union Crystal, Kynoch Press, Birmingham, England
Mao HK (1974) A discussion of the iron oxides at high pressure with implications for the chemical and thermal evolution of the earth. Ann Rep Geophys Lab 73:510–518
Mao HK, Bell PM (1971) High-pressure decomposition of spinel (Fe2SiO4). Ann Rep Geophys Lab 70:176–177
Mao HK, Takahashi T, Bassett WA, Weaver JS, Akimoto S (1969) Effect of pressure and temperature on the molar volumes of wüstite and of three (Fe, Mg)2SiO4 spinel solid solutions. J Geophys Res 74:1061–1069
Mizutani H, Hamano Y, Akimoto S, Nishizawa O (1972) Elasticity of stishovite and wüstite (abstr) Trans Am Geophys Union 53:527
Murnaghan FD (1951) Finite deformation of an elastic solid. Wiley, New York
Ringwood AE, Major A (1968) Apparatus for phase transformation studies at high pressures and temperatures. Phys Earth Planet Inter 1:164–168
Sawamoto H, Ohtani E, Kumazawa M (1974) High pressure decomposition of γ - Fe2SiO4. Proc 4th Int Conf High Press, Kyoto pp 194–201
Shen P, Bassett WA, Liu L (1983) Experimental determination of the effects of pressure and temperature on the stoichiometry and phase relations of wüstite. Geochim Cosmochim Acta 47:773–778
Simons B (1980) Composition-lattice parameter relationship of the magnesiowüstite solid solution series. Ann Rep Geophys Lab 79:376–380
Simons B, Seifert F (1979) High-pressure wüstite: cell parameters and Mössbauer spectra. Ann Rep Geophys Lab 78:625–626
Sumino Y, Anderson OL (1980) Elastic constants of single-crystal MgO at temperatures between 80° K and 1,300° K (abstr) Trans Am Geophys Union 61:1102
Sumino Y, Kumazawa M, Nishizawa O, Pluschkell W (1980) The elastic constants of single crystal Fe1-x O, MnO and CoO, and the elasticity of stoichiometric magnesiowüstite. J Phys Earth 28:475–495
Suzuki I, Okajima A, Seya K (1979) Thermal expansion of single-crystal manganosite. J Phys Earth 27:63–69
Swanson HE, Fuyat RK, Ugrinic GM (1955) Standard X-ray diffraction powder patterns. Nat Bur Stand (US) Circ No 539
Touzelin B (1974) High-temperature x-ray determination of iron monoxide lattice parameters under controlled atmosphere. Decomposition of iron monoxide between 25 and 570° C. Rev Hautes Temp Refract 11:219–229
Vallet P, Raccah P (1965) Thermodynamic properties of solid iron (II) oxide. Mem Sci Rev Met 62:1–29
Weaver JS, Takahashi T, Bassett WA (1971) Calculation of the P — V relation for sodium chloride up to 300 kilobars at 25° C. In: Lloyd EC (ed) Accurate Characterization of the high pressure environment. Nat Bur Stand (US) Spec Publ No 326, pp 189–199
Wells AF (1950) Structural inorganic chemistry, 2nd edn Oxford University Press, New York
Will G, Hinze E, Nuding W (1980) The compressibility of FeO measured by energy dispersive x-ray diffraction in a diamond anvil squeezer up to 200 kbar. Phys Chem Minerals 6:157–167
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McCammon, C.A., Liu, Lg. The effects of pressure and temperature on nonstoichiometric wüstite, FexO: The iron-rich phase boundary. Phys Chem Minerals 10, 106–113 (1984). https://doi.org/10.1007/BF00309644
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00309644