Summary
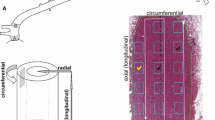
Rat mesenteric arteries, perfusion fixed in relaxed or contracted conditions, were digested with acid and elastase, bleach (sodium hypochlorite), or alkali to selectively remove collagen, elastin, or cells. Scanning electron microscopy was used to study the three-dimensional organization of the remaining cells or extracellular components. Smooth muscle cells of the tunica media were elongated and circumferentially oriented. Superior mesenteric artery cells had an irregular surface with numerous projections and some ends were forked. Small mesenteric artery cells were spindle shaped with longitudinal surface ridges, and showed extensive corrugations upon contraction. Elastin was present both as laminae and as an interconnected fibrous meshwork. Collagen was arranged in an irregular network of individual fibrils and small bundles of fibrils that formed nests around the cells in both arteries. This irregular arrangement persisted, with no apparent reordering or loss of order, upon contraction. The lack of an ordered arrangement or specialized organization at the cell ends suggests mechanical coupling of the cells to elastin or collagen throughout the length of the cell, allowing for force transmission in a number of directions. The tunica media is thus a “composite” material consisting of cells, elastin, and collagen. The isotropic network of fibers is well suited for transmitting the shearing forces placed on it by contraction of smooth muscle cells and by pressure-induced loading.
Similar content being viewed by others
References
Baluk P, Gabella G (1987) Scanning electron microscopy of the muscle coat of the guinea-pig small intestine. Cell Tissue Res 250:551–561
Blumenfeld OO, Bienkowski RS, Schwartz E, Seifter S (1983) Extracellular matrix of smooth muscle cells. In: Stephens NL (ed) Biochemistry of smooth muscle, vol 2. CRC Press, Boca Raton, Fla, pp 137–187
Chaldakov GN, Nara Y, Horie R, Yamori Y (1989) A new view of the arterial smooth muscle cells and autonomic nerve plexus by scanning lectron microscopy in spontaneously hypertensive rats. Exp Pathol 36:181–184
Chiesi M, Ho MM, Inesi G, Somlyo AV, Somlyo AP (1981) Primary role of sarcoplasmic reticulum in phasic contractile activation of cardiac myocytes with shunted myolemma. J Cell Biol 91:728–742
Clark JM, Glagov S (1979) Structural integration of the arterial wall. I Relationships and attachments of medial smooth muscle cells in normally distended and hyperdistended aortas. Lab Invest 40:587–602
Clark JM, Glagov S (1985) Transmural organization of the arterial media: the lamellar unit revisited. Arteriosclerosis 5:19–34
Dickson CP, Robinson TF (1988) Differentiating cardiac elastin, collagen and microfibrils with NaOH at the ultrastructural level. Histochemistry 89:105–107
Dobrin PB (1983) Vascular mechanics. In: Shepherd JT, Abboud FM, Geiger SR (eds) Handbook of physiology, sect 2: the cardiovascular system, vol 3. American Physiological Society, Bethesda, Md, pp 65–102
Drenckhahn D, Jeikowski H (1978) The myotendinous junction of the smooth feather muscles (mm pennati). Cell Tissue Res 194:151–162
Fay FS, Delise CM (1973) Contraction of isolated smooth-muscle cells: structural changes. Proc Natl Acad Sci USA 70:641–645
Fay FS, Fujiwara K, Rees DD, Fogarty KE (1983) Distribution of α-actinin in single isolated smooth muscle cells. J Cell Biol 96:783–795
Gabella G (1984) Smooth muscle cell membrane and allied structures. In: Stephens NL (ed) Smooth muscle contraction. Dekker, New York Basel, pp 21–45
Gabella G (1989) Structure of intestinal musculature. In: Handbook of physiology: sect 6: the gastrointestinal system. American Physiological Society, Bethesda, Md, pp 103–139
Gattone VH II, Miller BG, Evan AP (1986) Microvascular smooth muscle cell quantitation from scanning electron microscopic preparations. Anat Rec 216:443–447
Gerrity RG, Cliff WJ (1975) The aortic tunica media of the developing rat. I. Quantitative stereologic and biochemical analysis. Lab Invest 32:585–600
Hornebeck W, Tixier JM, Robert L (1986) Inducible adhesion of mesenchymal cells to elastic fibers: elastonectin. Proc Natl Acad Sci USA 83:5517–5520
Kan FWK (1990) Use of Peldri II as a sublimation dehydrant in place of critical-point drying in fracture-label cytochemistry and in backscattered electron imaging fracture-label. J Electron Microsc Tech 14:21–31
Kennedy JR, Williams RW, Gray JP (1989) Use of Peldri II (a fluorocarbon solid at room temperature) as an alternative to critical point drying for biological tissues. J Electron Microsc Tech 11:117–125
Kondo H, Ushiki T (1985) Polyethylene glycol (PEG) embedding and subsequent de-embedding as a method for the correlation of light microscopy, scanning electron microscopy, and transmission electron microscopy. J Electron Microsc Tech 2:457–462
Krizmanich WJ, Lee RMKW (1987) Scanning electron microscopy of vascular smooth muscle cells from rat muscular arteries. Scan Microsc 1:1749–1758
Lee RMKW, Triggle CR (1986) Morphometric study of mesenteric arteries from genetically hypertensive Dahl strain rats. Blood Vessels 23:199–224
Lee RMKW, Garfield RE, Forrest JB, Daniel EE (1979) The effects of fixation, dehydration and critical point drying on the size of cultured smooth muscle cells. Scan Electron Microsc 3:439–448
Lee RMKW, Forrest JB, Garfield RE, Daniel EE (1983a) Ultrastructural changes in mesenteric arteries from spontaneously hypertensive rats. Blood Vessels 20:72–91
Lee RMKW, Garfield RE, Forrest JB, Daniel EE (1983b) Morphometric study of structural changes in the mesenteric blood vessels of spontaneously hypertensive rats. Blood Vessels 20:57–71
Matthews MA, Gardner DL (1966) The fine structure of the mesenteric arteries of the rat. Angiology 17:902–928
Mecham RP, Hinek A, Griffin GL, Senior RM, Liotta LA (1989) The elastin receptor shows structural and functional similarities to the 67-kDa tumor cell laminin receptor. J Biol Chem 264:16652–16657
Mullins GL, Guntheroth WG (1965) A collagen net hypothesis for force transference of smooth muscle. Nature 206:592–594
Obara K (1984) Isolation and contractile properties of single smooth muscle cells from guinea pig taenia caeci. Jpn J Physiol 34:41–54
Ohtani O, Ushiki T, Taguchi T, Kikuta A (1988) Collagen fibrillar networks as skeletal frameworks: a demonstration by cell-maceration/scanning electron microscopic method. Arch Histol Cytol 51:249–261
Rhodin JAG (1980) Architecture of the vessel wall. In: Bohr DF, Somlyo AP, Sparks HV Jr, Geiger SR (eds) Handbook of physiology, sect 2: the cardiovascular system, vol 2: vascular smooth muscle. American Physiological Society, Bethesda, Md, pp 1–31
Roach MR, Song SH (1988) Arterial elastin as seen with scanning electron microscopy: a review. Scan Microsc 2:993–1004
Robert L, Jacob MP, Fulop T, Timar J, Hornebeck W (1989) Elastonectin and the elastin receptor. Pathol Biol 37:736–741
Somlyo AV (1980) Ultrastructure of vascular smooth muscle. In: Bohr DF, Somlyo AP, Sparks HV Jr, Geiger SR (eds) Handbook of physiology, sect 2: the cardiovascular system, vol 2: vascular smooth muscle. American Physiological Society, Bethesda, Md, pp 33–67
Street S (1983) Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol 114:346–364
Trotter JA, Corbett K, Avner BP (1981) Structure and function of the murine muscle-tendon junction. Anat Rec 201:293–302
Urry DW, Haynes B, Thomas D, Harris RD (1988) A method for fixation of elastin demonstrated by stress/strain characterization. Biochem Biophys Res Commun 151:686–692
Warshaw DM, McBride WJ, Work SS (1987) Corkscrew-like shortening in single smooth muscle cells. Science 236:1457–1459
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Walker-Caprioglio, H.M., Trotter, J.A., Mercure, J. et al. Organization of rat mesenteric artery after removal of cells or extracellular matrix components. Cell Tissue Res 264, 63–77 (1991). https://doi.org/10.1007/BF00305723
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00305723